Translate this page into:
Glomerulonephritis with Crescents in Polyomavirus Nephropathy
Address for correspondence: Dr. Aravind Sekar, Department of Histopathology, Post Graduate Institute of Medical Education and Research, Chandigarh, India. E-mail: aravindcmc88@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Polyomavirus nephropathy (PVN) is a known complication of renal transplantation due to the reactivation of latent BK virus (BKV) infection. Viral replication is usually confined to tubules. However, in severe viremia and late stages of PVN, it can involve glomerular parietal epithelial cells. Glomerular involvement by BKV can cause crescent formation and may lead to graft failure. We describe a relatively rare case of PVN with glomerular involvement and crescent formation in a 52-year-old male who had undergone a transplant 16 months ago. Despite the stoppage of immunosuppression, graft failure occurred eventually. Interestingly, we observed the intense positivity for IgG and c4d in the Bowman capsule on immunofluorescence. Observation of such positivity along Bowman capsule in renal biopsies with a limited number of glomeruli should alert pathologists to do a vigilant search of BKV inclusion and perform immunohistochemistry for SV 40 large T antigen.
Keywords
Crescents
graft failure
polyomavirus nephropathy
Introduction
Polyomavirus nephropathy (PVN) is an infectious complication of kidney transplantation due to the reactivation of latent BK virus (BKV) infection in the renal allografts.[1] It occurs in 5%–8% of renal allograft recipients due to intense immunosuppression, particularly after high doses of tacrolimus and mycophenolate mofetil.[2] PVN can occur as early as 1 month and as late as 10 years after renal transplantation. It is usually preceded by viremia after reactivation of latent virus infection. However, only 20%–40% of viremic patients develop morphologically apparent polyomavirus nephropathy.[34] Renal biopsy is considered the gold standard for the diagnosis of definitive PVN. The viral replication and nuclear changes are primarily seen in the epithelial cells of medullary collecting ducts and distal tubules during the early course of the disease.[5] In severe viremia and advanced stage, proximal tubular epithelial cells and glomerular parietal epithelial cells are also affected.[6] Sometimes, glomerular involvement by the BKV may cause the crescent formation and lead to rapid deterioration of renal function and graft loss.[7] We describe clinical, histological, and immunofluorescence findings of a relatively rare case of PVN with glomerular involvement leading to graft failure.
Case Report
A 52-year-old male with the primary disease of diabetic nephropathy had undergone a live emotionally related renal allograft transplant (LERRAT) 16 months ago. Induction therapy was not administrated. He was on triple immunosuppression (tacrolimus/mycophenolate mofetil/steroids). His serum creatinine levels were falling slowly and struck at 2.1 mg on the 6th day of the postoperative period. Biopsy was done and diagnosed as acute T-cell-mediated rejection. Three doses of intravenous methylprednisolone were given. Subsequently, serum creatinine level came to 1.4 mg/dl. He was on irregular follow-up. Three months ago, on investigation, he was found to have anemia (hemoglobin: 5.2 gm/dl) and deranged renal function (serum creatinine: 2.2 mg/dl). Peripheral blood smear showed dimorphic RBCs with both microcytes and macrocytes. He was transfused with two units of packed RBCs. Hemoglobin levels improved to 8.3 gm/dl. However, he continued to have easy fatiguability. His serum creatinine levels increased from 2.2 to 4.1 mg% over 1 month. There were no active sediments on urine analysis, and 24-h urine protein was 71 mg. Serum complements levels were within the normal limit. Antinuclear antibody testing by indirect immunofluorescence was negative. Blood tacrolimus level was 4.08 ng/ml. Renal biopsy was done for deranged renal function.
Light microscopy examination of the renal biopsy showed seven glomeruli, of which two were globally sclerosed. One glomerulus showed extra capillary hypercellularity with more than two-layer thickness and involving more than 10% of the circumference of the Bowman capsule, qualifying for cellular crescent. Cells forming the crescents showed enlarged nuclei containing ground glass intranuclear inclusion (Figure 1). A fibro-cellular crescent was observed in another glomerulus. There was no rupture of bowman capsule or glomerulitis or transplant glomerulopathy noted. The peritubular capillaries (PTC) did not show significant dilatation and margination of leukocytes. Tubular epithelial cells in 40% of tubules showed similar nuclear enlargement with amorphous ground glass intranuclear inclusions. It was accompanied by severe tubulitis (t3) and moderate interstitial inflammation (i2) comprising plasma cells and lymphocytes. There was moderate tubular atrophy and interstitial fibrosis. Immunohistochemistry (IHC) for SV40 large T antigen highlighted BK virus-infected tubular epithelial cells. Further, glomerular parietal cells in cellular crescentic glomeruli were also positive for SV 40 large T antigen, confirming glomerular involvement by BK virus. On immunofluorescence, the glomerular capillary wall and mesangium were negative for all immunoglobulins, complements, and fibrinogen. Peritubular capillaries were negative for c4d. However, moderate to intense positivity for IgG and c4d was noted in the tubular basement membrane and Bowman capsule. As per recent The Banff Working Group Classification, the index case was diagnosed and classified as polyomavirus nephropathy class III (pvl3; ci2) with glomerular involvement and crescent formation. Plasma PCR for BKV was 2.1 × 106 copies/ml.
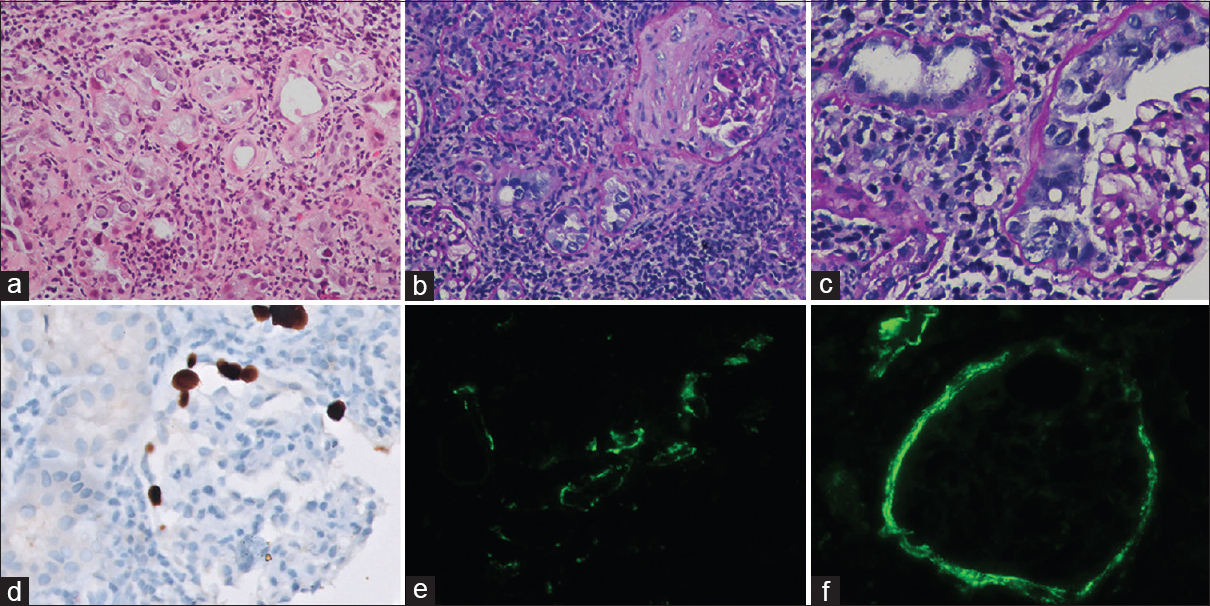
- Light microscopy of kidney biopsy showing amorphous ground glass intranuclear viral inclusions in tubular epithelial cells (a, Hematoxylin and Eosin, 200×), accompanied by severe tubulitis and moderate interstitial fibrosis with lympho-mononuclear inflammation (b, Periodic acid Schiff, 200×). Glomerulus showing BKV-induced cytopathic changes in proliferating parietal epithelial cells. (c, Periodic acid-Schiff, 400×). Immunohistochemistry for SV40 large T antigen highlighting BK virus-infected glomerular parietal cells (d, Immunoperoxidase, 400×). Immunofluorescence showing 2+ to 3+ intense C4d positivity along the tubular basement membrane (e, Fluorescein isothiocyanate, 200×) and Bowman capsule (f, Flavocytochrome, 200×)
The immunosuppression was cut down, and serum creatinine levels were monitored. However, serum creatinine progressively increased over months and resulted in graft failure. Further, the patient was lost to follow-up.
Discussion
Polyomavirus nephropathy is a significant complication of kidney transplantation due to the reactivation of latent polyomaviruses in the urogenital tract of the donor's kidney. Its incidence varies among transplant centers and is estimated to be 5%–8% in developed countries.[8] The incidence of PVN was higher in deceased donor renal allograft recipients, ABO-incompatible grafts, males, highly sensitized allograft recipients after desensitization, history of prior rejection, immunosuppressive protocol containing tacrolimus. Clinical presentation and histologic changes of PVN vary from case to case,[9] and in most cases, renal allograft biopsies are obtained in the setting of clinically unexplained deterioration of graft function.
Glomerular involvement and crescent formation are the relatively uncommon manifestation of PVN.[10] As the glomerular parietal epithelial cell is in direct continuity with tubular epithelial cells, it can be affected by BKV through ascending transmission in cases of severe viremia and late stages of PVN. In definitive PVN cases, glomerular infection by BKV and subsequent crescent formation were seen in 24.4% and 11.4%, respectively.[11] As in other conditions, the crescent formation in PVN can reduce single nephron GFR drastically and cause rapid deterioration of renal function.
In the index case, one cellular and fibro cellular crescents were seen. Viral inclusions and immunopositivity for SV40 large T antigen were noted in glomerulus with cellular crescent, proximal and distal tubular epithelial cells. Intrarenal viral load was very severe and was seen in 40% of tubules, classified as class III PVN (pvl3; ci2). Glomerulus with fibro cellular crescent did not show any viral inclusion or immunopositivity for SV40 large antigen. Crescents could be formed due to direct infection and subsequent proliferation of glomerular parietal cells.[12] Crescent formation due to recurrent or de novo immune complex-mediated glomerulonephritis was excluded as glomerular capillary and mesangium were negative for all immunoglobulins and complements.
On immunofluorescence, intense positivity for IgG and c4d was noted along the tubular basement membrane and Bowman capsule. This is probably due to the activation of the complement pathway by significant viremia and intrarenal viral load. A similar observation of Bowman capsule positivity for C4d was observed in cases of severe viremia by Batal et al.[13] C4d positivity in TBM is non-specific and can be seen in acute T-cell-mediated rejection without BKV infection. However, the Bowman capsule's positivity for c4d is specific, and such findings should prompt a more vigilant search for viral inclusions in the biopsy.
In the index case, glomerular involvement by BKV was observed only in one out of two cores. Thus, the diagnosis might be missed if only other non-diagnostic core is submitted for examination. In a study by Nickeleit et al.,[14] diagnostic PVN changes were limited to one core and medulla in 28% and 9% cases, respectively. Thus, as suggested by the Banff working group,[14] it is highly recommended that two biopsy cores, including renal medulla and IHC staining for SV 40 T large antigen, are required for definitive diagnosis of PVN and to document glomerular involvement by BK virus.
As there is no specific treatment for polyomavirus, patients with PVN have commonly been treated by reducing overall maintenance immunosuppression. The outcome of PVN is varied and depends on the histological class of PVN on renal biopsy at the time of presentation. Two factors, namely the intrarenal viral load and tubular atrophy with interstitial fibrosis, determine the PVN class. During the follow-up of 189 cases, 16% of grafts failed in PVN class 1, 31% of grafts failed in PVN class 2, and 50% of grafts failed in PVN class 3.
Conclusion
Glomerular involvement by BKV can lead to crescent formation and cause rapid deterioration of renal function. PVN should also be considered as one of the differential diagnoses in evaluating crescentic glomerulonephritis in renal allografts. Observation of Bowman capsule's positivity for c4d and IgG should alert the pathologist for vigilant search for BKV inclusions, utilize immunohistochemistry for SV40 large T antigen, and advise clinicians to perform plasma PCR testing.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- BK virus infection in a kidney allograft diagnosed by needle biopsy. Am J Kidney Dis. 1995;26:671-3.
- [Google Scholar]
- Polyomavirus disease under new immunosuppressive drugs: A cause of renal graft dysfunction and graft loss. Transplantation. 1999;67:918-22.
- [Google Scholar]
- Polyomaviruses and disease: Is there more to know than viremia and viruria? Curr Opin Organ Transplant. 2015;20:348-58.
- [Google Scholar]
- Polyomavirus infection of renal allograft recipients: From latent infection to manifest disease. J Am Soc Nephrol. 1999;10:1080-9.
- [Google Scholar]
- AJKD atlas of renal pathology: Polyomavirus nephropathy. Am J Kidney Dis. 2016;68:e37-8.
- [Google Scholar]
- Glomerular parietal epithelial cells infection is associated with poor graft outcome in kidney transplant recipients with BK polyomavirus-associated nephropathy. J Infect Dis. 2019;219:1879-86.
- [Google Scholar]
- Incidence and outcomes of BK virus allograft nephropathy among ABO- and HLA-incompatible kidney transplant recipients. Clin J Am Soc Nephrol. 2012;7:1320-7.
- [Google Scholar]
- BK virus nephropathy: Histological evolution by sequential pathology. Am J Transplant. 2017;17:2065-77.
- [Google Scholar]
- Pathological characteristics of BK polyomavirus-associated nephropathy with glomerular involvement. Ann Transl Med. 2020;8:923.
- [Google Scholar]
- The glomerular crescent: Triggers, evolution, resolution, and implications for therapy. Curr Opin Nephrol Hypertens. 2020;29:302-9.
- [Google Scholar]
- The significance of renal C4d staining in patients with BK viruria, viremia, and nephropathy. Mod Pathol. 2009;22:1468-76.
- [Google Scholar]
- The Banff Working Group Classification of Definitive Polyomavirus Nephropathy: Morphologic definitions and clinical correlations. J Am Soc Nephrol. 2018;29:680-93.
- [Google Scholar]







