Translate this page into:
Graves' disease in a dialysis dependent chronic renal failure patient
Address for correspondence: Dr. C. Gopalakrishnan Nair, Department of Surgery, Amrita School of Medicine, Kochi, Kerala, India. E-mail: gopalakrishnannair@aims.amrita.edu
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Thyroid hormone level may be altered in chronic renal failure patients. Low levels of thyroxine protect the body from excess protein loss by minimizing catabolism. Hyperthyroidism is rarely encountered in end-stage dialysis dependent patients. Less than 10 well-documented cases of Graves' disease (GD) are reported in literature so far. We report a case of GD in a patient on dialysis.
Keywords
Chronic renal failure
Graves' disease
hyperparathyroidism
hyperthyroidism
Introduction
Thyroid hormone levels drop down in starvation and in severe illness without detectable clinical effect of hypothyroidism. This condition was generally known as euthyroid sick syndrome, but was later renamed as nonthyroidal illness syndrome (NTIS).[1] NTIS is evidenced by low levels of thyroxines and normal or low levels of thyroid-stimulating hormone (TSH) and elevated reverse tri-iodothyronine (rT3) level. Clinical features of hypothyroidism do not develop within even 2-3 weeks, but require much longer period for expression.[2] Ramírez et al. studied patients receiving chronic hemodialysis and found a high prevalence of goiter (58%) and low serum tetra-iodothyronine (T4), T3, and TSH levels.[3] However, rT3 levels were not elevated in patients with end stage renal disease (ESRD).[4] The low levels of thyroid hormones may be due to decreased peripheral de-iodization of T4 and low levels of hormone binding proteins. The nonthyroidal illness serves to defend protein loss and an attempt to supplement hormone causes excess protein loss. The kidneys preserve iodine in patients suffering from ESRD and so inorganic iodine store of the body and the thyroidal iodine content are increased.[4] Prevalence of goiter and hypothyroidism was observed high in patients with ESRD compared with the general population in iodine deficient region.[5] Hyperthyroidism is uncommon in patients receiving dialysis, but sporadic case reports are available since 1980.[6]
Case Report
A 28-year-old female reported in March 2005 for evaluation of renal dysfunction noted during her first pregnancy. She developed bilateral pedal edema, hypertension and proteinuria during the third trimester of the first pregnancy in 1998. Thyroid evaluation during second and third trimester of pregnancy was reported to be normal. She underwent Caesarean section at 8th month of gestation, indication being accelerated hypertension. On evaluation 2 months after delivery, she was found to have persistent hypertension, proteinuria, and elevated serum creatinine (1.72 mg/dl). Tests for HIV, hepatitis B and C were negative. Screening tests for systemic lupus erythematosus were found negative. Kidney biopsy showed focal glomerular sclerosis with an increase in the mesangial matrix and tubular atrophy, suggestive of focal segmental glomerulosclerosis. She was treated with oral prednisolone, but developed pain in both hip joints after 5 months. Regional magnetic resonance imaging showed avascular necrosis of left femur head and so prednisolone was tapered and discontinued. She underwent hemiarthroplasty of left hip. Despite supportive treatment, kidney function deteriorated steadily and in 2005, she was initiated hemodialysis. Thyroid hormone levels were normal [Table 1].

In 2009, she experienced a sudden loss of weight (4 kg over 4 months). Clinical examination revealed body mass index 18.59 kg/m2, with Grade I diffuse enlargement of thyroid gland and Grade II inactive orbitopathy. Laboratory studies indicated hyperthyroidism [Table 1]. Technetium 99 thyroid scintigraphy showed diffuse uptake of 6.8% (normal 1-4%) indicating Graves' disease (GD) [Figure 1].
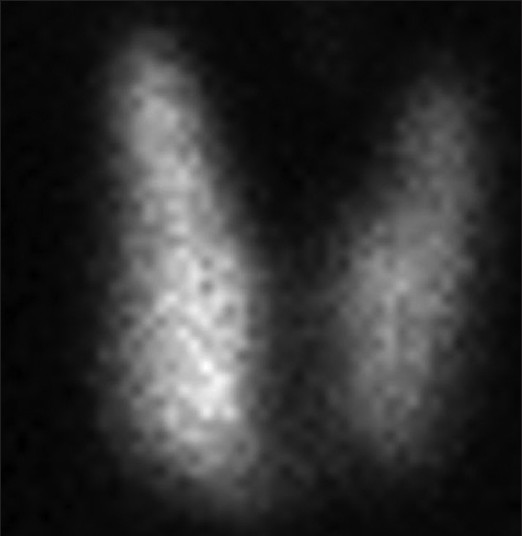
- Technetium pertechnetate thyroid thyroid scintigraphy
She was placed on carbimazole (20 mg daily as a single dose) and propranolol (20 mg twice daily). Propranolol was discontinued since she developed dizziness and postural hypotension. She became biochemically euthyroid after 4 months of antithyroid drugs. She developed retrobulbar pain and redness of eyes while on carbimazole. Examination revealed Grade II active orbitopathy, which was controlled by symptomatic treatment. The laboratory investigation results are noted in Table 1.
She developed relapse of hyperthyroidism upon weaning carbimazole after 14 months, and total thyroidectomy was offered. I-131 ablation was not planned considering the deteriorating orbital lesion. She preferred to remain on carbimazole (10 mg daily). She developed generalized body pain, asthenia and muscle weakness suggestive of myopathy and became wheel chair bound by 2012. Bone mineral densitometry studies of right wrist and lumbar spines showed T score of −3 and −2.7, respectively. She was diagnosed to have secondary hyperparathyroidism [Figure 2].
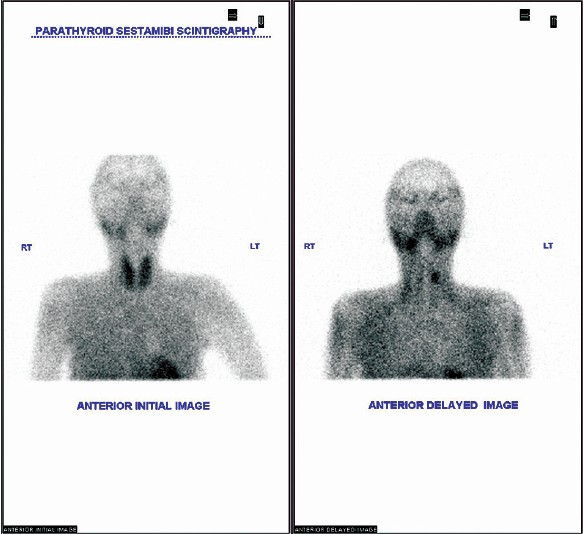
- Technetium MIBI double phase parathyroid scintigraph
After control of thyrotoxicosis, total thyroidectomy and subtotal parathyroidectomy retaining portion of right superior parathyroid gland were done. Thyroid gland was diffusely enlarged with nodules in the right lobe and weighed 49 g. Histology showed hyperplasic follicles lined by occasional tall follicular cells. There was scanty pale colloid and occasional florid papillary hyperplasia. There was dense infiltration of lymphocytes in the stroma. The final histology showed features suggestive of GD of the thyroid gland and hyperplasia of parathyroid glands. The patient had uneventful postoperative recovery and symptomatic relief.
Discussion
Hyperthyroidism is rare in patients on dialysis. The first case was reported in 1980[6] but later about 18 such cases were reported.[7] However, Boubakera et al. in a comprehensive review noted that only eight well documented cases of GD were detected in ESRD patients.[8] The present case demonstrated clinical features of hyperthyroidism with diffuse goiter and orbital involvement suggesting an autoimmune etiology. The Tc99 scintigraphy also noted excess uniform concentration of the radiopharmaceutical.
The clinical diagnosis of hyperthyroidism in ESRD may be delayed due to overlap of symptoms. In suspected patients close follow-up and frequent thyroid hormone estimation are advisable.
The serum iodine level as well as intrathyroidal iodine content can be high in ESRD patients and likely to exert transient or extended Wolf-Chaikoff phenomenon.[48] The excess iodine may induce thyroiditis resulting in hypothyroidism. On the other hand, whether the excess iodine stimulates the gland to a hyperactive state (Jod-Basedow effect) is not clear.
All documented cases were successfully treated with I-131 ablation (7-10). Carbimazole was used in this patient after dialysis on those days since the drug could be washed off during the procedure. High dose of carbimazole was recommended since failure of conversion to active form is likely due to metabolic acidosis.[9] However, we observed that euthyroid state could be achieved without dosage adjustment.
Surgical treatment was planned in this patient due to the presence of active orbitopathy and secondary hyperparathyroidism. All the more use of glucocorticoids was not advisable in case of deterioration of orbitopathy. Patient withstood the operation without any untoward events during the procedure or afterwards.
Patient had persistent and resistant anemia, which improved after thyroidectomy. Hyperthyroidism may also contribute to anemia in ESRD patients.[10]
Source of Support: Nil
Conflict of Interest: None declared.
References
- Dangerous dogmas in medicine: The nonthyroidal illness syndrome. J Clin Endocrinol Metab. 1999;84:151-64.
- [Google Scholar]
- Differential end organ responsiveness to suboptimal thyroid hormone concentrations as assessed by short-term withdrawal of levothyroxine sodium in athyreotic patients. In: Proceeding 70th Annual Meeting of the American Thyroid Association. Colorado Springs, CO. :S-88.
- [Google Scholar]
- Thyroid abnormalities in renal failure. A study of 53 patients on chronic hemodialysis. Ann Intern Med. 1973;79:500-4.
- [Google Scholar]
- Thyroid function in patients with chronic renal failure. Am J Kidney Dis. 2001;38(4 Suppl 1):S80-4.
- [Google Scholar]
- Thyroid disorders in hemodialysis patients in an iodine-deficient community. Artif Organs. 2005;29:329-32.
- [Google Scholar]
- Clinical hyperthyroidism in a patient receiving long-term hemodialysis. Arch Intern Med. 1980;140:708-9.
- [Google Scholar]
- Management of hyperthyroidism with radioactive iodine in end-stage renal disease patients undergoing dialysis. Pol Arch Med Wewn. 2004;112:931-6.
- [Google Scholar]
- Characteristics of Graves' disease in haemodialysis patients. J Endocrinol Metab. 2012;2:212-5.
- [Google Scholar]
- Carbimazole therapy in the setting of end-stage renal disease and haemodialysis. Nephrol Dial Transplant. 2006;21:2318-9.
- [Google Scholar]
- An unusual etiology of erythropoietin resistance: Hyperthyroidism. Ren Fail. 2007;29:759-61.
- [Google Scholar]







