Translate this page into:
Heavy Metals and Pesticides in Chronic Kidney Disease – Results from a Matched Case–Control Study from a Rural Population in Shivamogga District in South India
Address for correspondence: Dr. Y. J. Anupama, Nanjappa Hospital, Shivamogga - 577 201, Karnataka, India. E-mail: anupamayj@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
There is a high prevalence of chronic kidney disease (CKD) in the rural agrarian population of South India and it often appears unrelated to major known causes such as diabetes or glomerulonephritis.
Methods:
In a matched case–control study conducted in a rural population in Shivamogga district in South India, the association of heavy metals – lead (Pb), arsenic (As), cadmium (Cd) – and pesticides in CKD was studied. Blood and spot urine samples were tested quantitatively for heavy metals and qualitatively for pesticides.
Results:
In all, 69 matched pairs (40 female, 58%) were recruited. The mean estimated glomerular filtration rate (mL/min/1.73 m2) was 60.1 (14.2) in cases and 83.4 (13.4) in controls. Elevated blood lead level >5 μg/dL was seen in 15 cases and 25 controls, respectively [P = 0.035, matched odds ratio (MOR) 0.5, 95% confidence interval (CI) 0.22–1.05]. Urinary Pb was elevated in 16 cases and 13 controls, respectively (P = 0.28, MOR 1.25, 95% CI 0.58–2.73). There was no significant association with As and Cd, while pesticide residues were undetectable in cases as well as controls. These results did not change even after excluding CKD cases with diabetes, stage 2 hypertension, and significant proteinuria.
Conclusions:
There was no statistical significant association between any of the studied heavy metals and CKD, although there was a significant burden of heavy metals in the studied subjects.
Keywords
Chronic kidney disease
epidemiological study
heavy metals
India
matched case–control study
rural population
Introduction
Chronic kidney disease (CKD) is widely acknowledged to be a global epidemic.[1] The global prevalence is on the rise and is estimated to be between 11% and 13%.[2] The rise in prevalence is not only seen in the developed countries but also seen in the developing countries. The cause for this appears to be multifactorial. The changing lifestyle among the people in these countries due to socioeconomic transition and the ageing trend of the population have led to an increase in comorbidities such as diabetes, hypertension, obesity, and cardiovascular diseases that have directly contributed to the CKD burden.[3] Factors such as poor sanitation, overcrowding, illiteracy, infections, low socioeconomic status, and poor reach of quality medical services to remote areas have also resulted in an increase in the CKD prevalence in these countries.[45] CKD of unknown etiology (CKDu) is a new disease entity seen in some of the developing countries, predominantly affecting the population dependent on agriculture. Mesoamerican nephropathy in the Central American countries and Sri Lankan nephropathy in Sri Lanka are the well-described disease prototypes.[67] They have in common a chronic tubulointerstitial disease pattern and are etiologically linked to toxins in the environment.[891011] Heavy indiscriminate use of agrochemicals with consequent toxic contamination of the drinking water and food with heavy metals and heat stress are some of the causes incriminated.[121314]
In India, CKDu similar to these prototypes has been described from the Uddanam area of Andhra Pradesh.[15] However, it may be more widespread than previously believed. In a previous study done on the rural population of Shivamogga district in South India, more than 40% of the subjects had minimal or no proteinuria and mild to moderate hypertension which raised the possibility of CKDu.[16] In a subsequent follow-up conducted in 2014–2015 also, a similar trend was observed.[17] A preliminary evaluation for heavy metals revealed increased levels of urinary lead in some of these subjects (author's unpublished observations). Hence, this study was undertaken to systematically evaluate the levels in blood and urine for heavy metals such as lead (Pb), arsenic (As), cadmium (Cd) in patients with CKD and to analyze whether there was any association between the two. The study also looked at the pesticide residues and their association with CKD.
Methods
Study setting and study design
This matched case–control study was conducted in 2015–2016 on the adult population of Hosakoppa, Indiranagar, and Gajanur villages of Shimoga district, Karnataka state in South India. The villages are located about 10 km from Shivamogga town, with agriculture being an important occupation. The villages had been screened on two previous occasions, in 2011–2012[16] and again in 2014–2015.[17] All the persons who were identified as patients with CKD in these previous studies were approached for recruitment. The consenting individuals were reevaluated and recruited as cases in this study, after confirming the diagnosis of CKD. We recruited 69 cases after excluding those subjects who were not available for interview even after visits to their houses on two different days and those subjects who had relocated or did not consent to the study. A list of subjects who did not have CKD as found in the earlier studies was prepared. Line list of these subjects who were matched with cases for age (+ or − 4 years), gender, and village was finalized. Controls were selected from this line list using random number tables generated by OpenEpi version 3.1.[18] If a selected control had relocated, died, or was not available for interview even after visits to his house on two different days, the subject next in the list was selected as a matched control.
Study population
All adults (age 18 years and above) were considered for inclusion in the study. Elderly people >75 years of age, pregnant women, and women in the postnatal period (up to 6 weeks after delivery) were excluded. Cases of CKD in stage 5 already receiving dialysis and renal transplant recipients were also excluded.
Data collection and tools
The study was conducted from August 2015 to May 2016. The study was approved by the Institutional Ethics Committee. A written, informed consent was taken from each participant. The field work was done by one of the authors (SKK) along with the students of the nursing college affiliated to the investigating institution. All participants were administered a structured questionnaire, which included demographic details such as educational and occupational status, living conditions, and personal habits such as smoking and alcohol use. History of diabetes, hypertension, renal disease, ischemic heart disease, stroke, and arthritis was elicited. Details of current medications, long-standing analgesic use, and indigenous medicine intake were as recorded. Weight, height, waist circumference, and hip circumference were measured as per standard protocol. Waist/hip ratio and body mass index were calculated. Blood pressure (BP) measurements were taken for the entire group using mercury sphygmomanometers (Diamond™, Pune, India), which were regularly calibrated. Two measurements were taken in the sitting position 5 min apart with the arms resting on a surface and the average was considered to be the BP.
Blood samples were collected for the following tests: hemoglobin, glycosylated hemoglobin (HbA1C), serum creatinine, and uric acid. Blood samples were analyzed in the parent institution using a fully automated biochemistry analyzer (A-25; Biosystems SA, Barcelona, Spain). HbA1C was tested by high-performance liquid chromatography method using Bio-Rad D10 analyzer (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Serum creatinine was analyzed by the Jaffe's method using standards and reagents (Biosystems SA) which are traceable to isotope dilution mass spectroscopy. Uric acid was analyzed using glucose oxidase method. Glomerular filtration rate (GFR) was estimated in all subjects using the Modification of Diet in Renal Diseases (MDRD) study equation. Random midstream urine samples were assessed using 11-parameter dipstick (Agappe Diagnostics, Ernakulam, Kerala, India) for urine protein, sugar, blood, and macroalbuminuria (MA) mechanically read by urine analyzer (MispaUriskan 100; Agappe Diagnostics, Ernakulam, Kerala, India). In all cases, albuminuria was also examined by semi-quantitative estimation of urinary albumin creatinine ratio (ACR) using Siemens Clinitek dipstick (Siemens Healthcare GmBH, Erlangen, Germany). It was mechanically read by BiosenseUchek, a smartphone-based portable diagnostic system (Biosense Diagnostics, Thane, Maharashtra, India). Quantitative analysis of urinary Beta-2 microglobulin (β-2), was done by immunoturbidimetric method using Beta-2 m turbilatex kit (Spin-React, SA, Sant Esteve De Bas, Spain). Care was taken to transport all blood and urine samples to the laboratory in cold chain and process them immediately.
Toxicology analysis
Toxicological analysis was done in a national-accredited toxicology laboratory at Amrita Institute of Medical Sciences, Cochin, India. Precautions were taken to avoid contamination of the samples during collection and transport. Blood and urine were analyzed quantitatively for lead, arsenic, and cadmium by inductively coupled plasma atomic energy spectrometry method (Iris Intrepid II XSP Duo; Thermo Electron Corporation, Madison, WI, USA). Samples were also tested qualitatively by thin-layer chromatography for residues of a panel of commonly used pesticides. The tested compounds included malathion, methyl parathion, quinalphos, monocrotophos, chlorpyriphos, carbofuran, carbaryl, carbendazim, propoxur, lindane, DDT, cypermethrin, permethrin, prallethrin, and imidacloprid.
Definitions of relevant parameters in the study
The case of CKD was defined as an adult residing in the villages mentioned above who had evidence of CKD. CKD was defined as the presence of either kidney damage or GFR <60 mL/min/1.73 m2.[19] Persistent albuminuria, defined as urine positive for MA on dipstick and spot ACR ≥30 mg/g, was taken as the indicator of kidney damage. Proteinuria was detected by the presence of protein in urine as indicated by 1+ (0.3 g/L) or more on dipstick.[20] Adult residing in the above-mentioned villages, who had no evidence of CKD, was considered to be the control.
Hypertension was defined as the presence of systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg or self-reported history of hypertension or use of antihypertensive medications.[21] Diabetes mellitus was defined as HbA1C ≥6.5% or self-reported history of diabetes or taking antidiabetes medications.[22] BMI was staged using the World Health Organization Asia Pacific criteria: malnutrition <18.5 kg/m2, normal BMI 18.5–22.9 kg/m2, overweight >23 kg/m2, and obesity >25 kg/m2.[23] Abdominal obesity was identified by the criteria of WC in men >90 cm and women >80 cm.[23] Urinary β-2 microglobulin levels were considered elevated when the levels exceeded 0.3 μg/mL.[24]
Heavy metals and pesticides: Elevated blood lead level (BLL) was defined as blood lead more than 5 μg/dL. Blood cadmium levels >6 μg/L and blood arsenic level >12 μg/L were considered elevated. Urinary lead, cadmium, and arsenic levels were considered elevated when spot urine lead, cadmium, and arsenic levels were more than 4, 1.3, and 35 μg/L, respectively.[25]
Statistical analysis
Baseline descriptive data are presented as proportions, mean with standard deviation, and median as applicable to individual variables. Comparative data by groups are described as mean and 95% confidence intervals (CIs). Student's t-test or Mann–Whitney U-tests were used to compare the variables between the two groups depending on the normality of the data. SPSS version 16 (SPSS, Inc., Chicago, IL, USA) was used to perform above analysis. Concordance and discordance among pairs for each variable of interest were manually identified and entered into paired matched odds ratio (MOR) contingency tables, using OpenEPI version 3.01 software, and mid P exact values and MOR were obtained. Two-sided P value of <0.05 and MOR >1 with 95%confidence intervals were accepted as statistically significant. For analyzing blood and urine heavy metal levels, the values reported to be below detectable limits were censored to calculate means and medians. Pearson's product moment correlation was determined to assess correlation between heavy metals and estimated GFR (eGFR) and also with urinary β-2 microglobulin levels.
Results
Baseline characteristics
In all, 69 pairs of cases and controls were recruited. There were 40 (58%) female pairs and 29 (42%) male pairs. The mean [±SD] ages of cases and controls were 49.2 ± 13.8 and 48.9 ± 13.3 years, respectively. The sociodemographic characteristics of cases and controls are summarized in Table 1.
| Characteristic | Cases, n=69 | % | Controls, n=69 | % |
|---|---|---|---|---|
| Age (years), mean (SD) | 49.2 (13.8) | NA | 49.04 (13.46) | NA |
| Males, no. (%) | 29 | 42.0 | 29 | 42.0 |
| Education status - elementary school | 58 | 84.1 | 60 | 87.0 |
| Occupation: agriculture | 28 | 40.6 | 21 | 30.4 |
| Occupation: manual labor | 16 | 23.2 | 32 | 46.4 |
| Nonvegetarian food habits | 60 | 87.0 | 57 | 82.6 |
| Smoking | 15 | 21.7 | 6 | 8.7 |
| Chewing of tobacco | 19 | 27.5 | 13 | 18.8 |
| Alcohol | 31 | 44.9 | 25 | 36.2 |
| Self-reported diabetes | 8 | 11.6 | 6 | 8.7 |
| Self-reported hypertension | 24 | 34.8 | 10 | 14.5 |
| Self-reported history of renal stones | 2 | 2.9 | 3 | 4.3 |
SD: Standard deviation; NA: Not applicable
Analysis of cases and controls
Clinical characteristics of cases and controls are summarized in Table 2. The mean serum creatinine was 1.15 ± 0.26 mg/dl in cases and 0.85 ± 0.14 mg/dl in controls (P < 0.001). The mean eGFR was 60.12 ± 14.28 ml/min in cases and 83.09 ± 12.97ml/min in controls (P < 0.001). Albuminuria was seen in 47 (68%) and 6 (8.7%) had significant proteinuria. Stagewise distribution of the CKD cases was as follows: stage 1: 2.9%, stage 2: 43%, stage 3a: 40%, and stage 3b: 13%. There were no patients in stage 4 CKD. One-fourth of our study population were diabetics (cases: n = 13, 19%; controls: n = 15, 21%, P = 0.81). In all, 59% of cases (n = 41) and 43% of controls (n = 30) were found to be hypertensive (P = 0.03). The majority of the study subjects had BMI in the normal range, while 23% had BMI >25 kg/m2. Abdominal obesity was seen in 44%. There were no significant differences between the two groups when means of variables were examined, apart from serum creatinine and eGFR [Table 2]. However, one important observation was that 27 participants had significant elevation of urinary β-2 microglobulin, of whom 23 (85%) were cases and only 4 (15%) were controls. The mean and median urinary β-2 microglobulin in the cases were 0.85 ± 1.32 and 0.13 μg/mL, respectively, while that in controls was 0.09 ± 0.14 and 0.04 μg/mL, respectively (P < 0.001).
| Characteristic, mean (SD) | Cases, n=69 | Controls, n=69 | P | 95% CI |
|---|---|---|---|---|
| Height, cm | 155.4 (8.05) | 156.7 (1.1) | 0.374 | −4.24, 1.60 |
| Weight, kg | 53.9 (4.2) | 53.1 (12.0) | 0.677 | −3.01, 4.61 |
| Body mass index, kg/m2 | 22.2 (4.3) | 21.6 (3.76) | 0.317 | −0.66, 2.04 |
| Hip circumference, cm | 91.14 (10.43) | 89.52 (8.34) | 0.315 | −1.55, 4.80 |
| Waist circumference, cm | 82.44 (12.25) | 80.91 (8.34) | 0.459 | −2.55, 5.63 |
| Waist/hip ratio | 0.90 (0.076) | 0.89 (0.079) | 0.321 | −4.22, 1.39 |
| Systolic blood pressure, mmHg | 135.72 (22.69) | 130.58 (17.93) | 0.106 | −1.21, 12.55 |
| Diastolic blood pressure, mmHg | 84.72 (14.27) | 83.13 (8.59) | 0.428 | −2.37, 5.56 |
| Hemoglobin, g/dL | 12.58 (2.62) | 12.24 (1.91) | 0.39 | −0.43, 1.11 |
| Glycosylated hemoglobin, % | 5.94 (1.21) | 5.83 (1.29) | 0.612 | −0.31, 0.53 |
| Serum creatinine, mg/dL | 1.15 (0.26) | 0.85 (0.14) | <0.001 | 0.22, 0.36 |
| Serum uric acid, mg/dL | 3.87 (1.15) | 4.18 (1.13) | 0.108 | −0.70, 0.07 |
| Estimated GFR, MDRD mL/min/1.73 m2 | 60.12 (14.28) | 83.09 (12.97) | <0.001 | −27.56, −18.37 |
SD: Standard deviation; CI: Confidence interval; GFR: Glomerular filtration rate; MDRD: Modification of Diet in Renal Diseases study equation. Significant difference marked in bold
Toxicological analysis
Blood lead and urinary lead levels were high in a significant number of study participants [Figures 1 and 2]. In all, 40% of the participants (27% cases and 50% controls) had elevated BLL [Table 3]. Nearly 75% of them had blood lead above >5 μg/dL. Urine lead was elevated in 21% of participants (23% cases and 19% controls). The median BLL and ULL was 22.25 and 178 μg/L, respectively. However, they did not differ significantly between cases and controls. A few of the subjects had blood and urine cadmium and arsenic too. Urine arsenic was elevated in 11% of the subjects (9% cases and 13% controls). The median level of urine arsenic was significantly higher in cases (501 μg/L) than in controls (233.5 μg/L; P = 0.04) [Figure 3]. However, there was no significant association between cases and controls with any of the heavy metals [Table 4]. Pearson's product moment correlation coefficients generated for heavy metals with eGFR also suggest that there is a positive correlation between the heavy metals but not with eGFR [Table 5]. There was no significant correlation of studied heavy metal levels with urinary β-2 microglobulins as well [Table 6]. Pesticide residues were not detected in either cases or controls by the method studied.
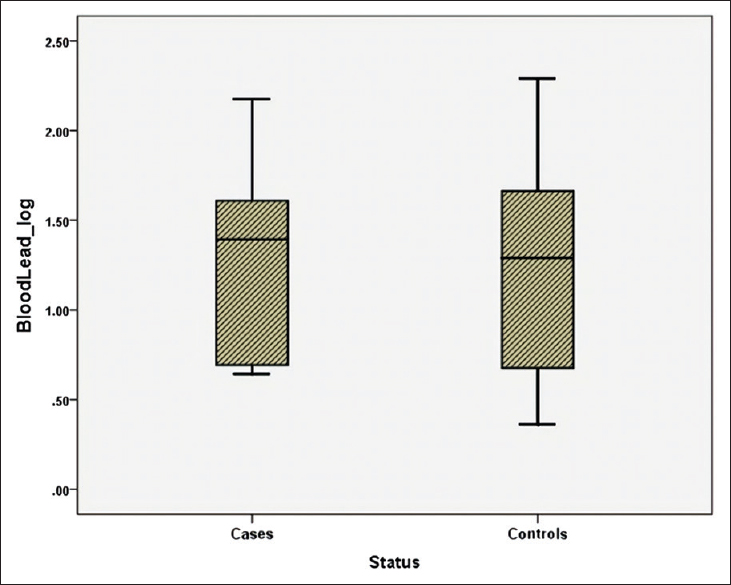
- Blood lead levels among cases and controls

- Urine lead levels among cases and controls
| Heavy metals | Statistical measure | Cases, n=69 | Controls, n=69 |
|---|---|---|---|
| Blood lead | Detectable values, number | 19 | 35 |
| Range | 4.4-155.1 | 2.3-195.1 | |
| Significant (>5 µg/dL), number | 15 | 25 | |
| Mean* (SD) | 35.6 (39.3) | 34.5 (40.9) | |
| Median (IQR)* | 24.7 (38.7) | 19.5 (41.8) | |
| P** | 0.69 | ||
| Urine lead, µg/L | Detectable values, number | 16 | 13 |
| Range | 24.2-1200 | 24637 | |
| Mean* (SD) | 342.9 (380.4) | 234.7 (398.3) | |
| Median (IQR)* | 227.2 (467.2) | 142.6 (398.3) | |
| P** | 0.661 | ||
| Blood arsenic, µg/L | Detectable values, number | 3 | 2 |
| Range# | 101 | 101 | |
| Mean* | 101 | 101 | |
| SD | NA | NA | |
| Median (IQR)* | NA | NA | |
| Urine arsenic, µg/L | Detectable values, number | 6 | 9 |
| Range | 448-501 | 44.4-440.6 | |
| Mean* (SD) | 483.5 (27.1) | 216.4 (136.4) | |
| Median (IQR)* | 501 (52.3) | 233.5 (224) | |
| P** | 0.001 | ||
| Blood cadmium, µg/L | Detectable values, number | 1 | 6 |
| Range | 44.6 | 4.5-69.2 | |
| Mean* (SD) | NA | 43.4 (21.2) | |
| Median (IQR)* | NA | 46.5 (20.8) | |
| Urine cadmium, µg/L | Detectable values, number | 1 | 5 |
| Range | 54.5 | 25.9-178.9 | |
| Mean* (SD) | NA | 67.7 (62.6) | |
| Median (IQR)* | NA | 44.8 (77) | |
SD: Standard deviation; CI: Confidence interval; IQR: Interquartile range; NA: not applicable. *Derived after censoring undetectable values; **derived for difference between rank sum of the two groups; #all subjects had the same value; significant difference indicated in bold

- Urine arsenic among cases and controls
| Heavy metals | Cases, no. (n=69) | % | Controls, no. (n=69) | % | P | MOR | 95% CI |
|---|---|---|---|---|---|---|---|
| Blood lead >5 mcg/dL | 15 | 21.74 | 25 | 36.23 | 0.061 | 0.47 | 0.20, 1.03 |
| Urine lead | 16 | 23.19 | 13 | 18.84 | 0.571 | 1.25 | 0.58, 2.73 |
| Blood arsenic | 3 | 4.35 | 2 | 2.90 | >0.991 | 1.50 | 0.22, 12.61 |
| Urine arsenic | 6 | 8.70 | 9 | 13.04 | 0.424 | 0.62 | 0.18, 1.93 |
| Blood cadmium | 1 | 1.45 | 6 | 8.70 | 0.071 | 0.16 | 0.01, 1.12 |
| Urine cadmium | 1 | 1.45 | 5 | 7.25 | 0.125 | 0.20 | 0.01, 1.44 |
MOR: Matched odds ratio; CI: Confidence interval
| Heavy metals | Correlation with metals | Pearson’s correlation | Sig. (two-tailed) | Pearson’s correlation with estimated GFR | Sig. (two-tailed) |
|---|---|---|---|---|---|
| Blood lead | Urine lead | 0.101 | 0.239 | 0.061 | 0.477 |
| Blood arsenic | 0.439** | <0.001 | |||
| Urine lead | Blood cadmium | 0.263** | 0.002 | −0.016 | 0.85 |
| Urine cadmium | 0.195* | 0.022 | |||
| Blood arsenic | Urine lead | −0.064 | 0.453 | −0.105 | 0.221 |
| Urine arsenic | 0.484** | <0.001 | |||
| Urine arsenic | Blood cadmium | −0.041 | 0.631 | −0.079 | 0.357 |
| Urine cadmium | −0.032 | 0.707 | |||
| Blood cadmium | Urine cadmium | 0.521** | <0.001 | 0.017 | 0.841 |
| Urine cadmium | Urine lead | 0.195* | 0.022 | 0.092 | 0.285 |
GFR: Glomerular filtration rate. **Correlation is significant at the 0.01 level (two-tailed); *correlation is significant at the 0.05 level (two-tailed); significant correlations denoted in bold
| Variable | Pearson’s correlation | Significance |
|---|---|---|
| Blood lead | 0.12 | 0.892 |
| Urine lead | −0.083 | 0.381 |
| Urine arsenic | −0.112 | 0.192 |
| Estimated GFR | −0.384** | <0.001 |
| Urinary protein | 0.259** | 0.002 |
| Microalbuminuria | 0.306** | <0.001 |
GFR: Glomerular filtration rate. **Correlation is significant at the 0.01 level (two-tailed); Please note: correlation not checked for blood arsenic and cadmium as the positive samples are very few in number; significant correlations denoted in bold
Subgroup analysis
We identified those cases without diabetes, significant hypertension (BP ≥160/110 mmHg), overt proteinuria (>0.3 g/L), and self reported kidney stones and considered them to be having CKD of undetermined etiology (CKDUD). A pair matched analysis for all the variables including heavy metals with their respective controls did not show significant association between the heavy metals and CKDUD.
Discussion
In this study, we found that our patients with CKD had significant urinary excretion of β-2 microglobulin even when they did not have significant proteinuria or albuminuria. There was significant lead burden as demonstrated by high blood and urine lead levels in the study population. A few subjects also had high levels of arsenic and cadmium. However, in our limited study, we could not demonstrate any significant positive association between these heavy metals and CKD.
In India, CKDu has been described from the coastal belt of Andhra Pradesh and Odisha states. Our study suggests that CKDu may be more widespread in India than previously thought. We had earlier reported a high prevalence of nonproteinuric CKD in agricultural workers in a previous study conducted in the same area in Karnataka state in South India.[16] In this study too, 32% cases had urinary ACR <30 mg/g. Furthermore, our cases had significant urinary excretion of β-2 microglobulin. Increased urinary excretion of low molecular weight proteins such as β-2 microglobulin is a feature of most toxic nephropathies.[2627] It indicates proximal tubular dysfunction and has been described in lead nephropathy and nephrotoxicity due to cadmium, arsenic, and mercury.[28] Low-molecular-weight proteinuria has also been described in CKDu as seen in Sri Lanka and the Central American countries and it is likely that our patients too have similar tubulointerstitial pattern of kidney damage. However, in our limited sample, we did not find a significant correlation of urinary β-2 microglobulin with the studied heavy metal levels in either blood or urine.
Studies in Sri Lanka point to a possible link between environmental toxins and CKDu. Various studies have demonstrated increased urinary excretion of As, Cd, and glyphosate in cases with CKDu.[293031] The source of these metals may be agrochemicals or other environmental sources such as well water, rice, fish, and pesticides.[32333435] However, studies on possible environmental toxic exposures carried out in the coastal belt of Andhra Pradesh in India have not reported a positive association with either agrochemicals or heavy metals till date.[3637] In our study too, we could not demonstrate significant association between CKD and heavy metals in either blood or urine. It is possible that we could not demonstrate a positive association between the heavy metal levels and CKD because our study patients had milder renal disease compared with the subjects in the Sri Lankan studies.[1438] It is possible that the effect of these metals may be better demonstrable as renal functions decline further. Interactions between metals or genetic polymorphisms also may influence effect of the metals on the kidneys.[3940]
To the best of our knowledge, this is a first ever matched case–control study from India that studied the association of CKD with heavy metals and pesticides. Another positive aspect of our study is the finding of significant urinary excretion of β-2 microglobulin in CKD cases that probably indicates a tubulointerstitial pattern of kidney damage. This is another finding that has not been demonstrated in any Indian study on CKD till date.
The high lead burden in the study subjects is alarming. Our study showed that nearly 40% of the study participants have elevated BLLs. Spot urine lead was elevated in about 20% of the participants. The magnitude of the blood and urine lead levels is large and far exceeds the range of lead levels seen in those studies which were done on occupationally lead-exposed subjects. This has enormous public health implications and needs further preventive measures.
There are some limitations with our study. It would have been more accurate to report urinary heavy metal levels corrected to urinary creatinine values which we did not do in our analysis. A few Sri Lankan studies have reported higher uncorrected urinary heavy metal levels in controls than in cases, but when the levels were corrected to urinary creatinine, the levels were significantly higher in cases than in controls.[1438] It would have been desirable to test the subjects for pesticide residues using quantitative methods. Third, the small sample size limits the generalizability of the findings and calls for larger study in this direction.
In summary, this study showed that there is no demonstrable association between CKD and heavy metals in this part of rural area of Shivamogga district in South India. A significant lead burden among the study participants and high levels of arsenic and cadmium in a few participants have been detected. This calls for more studies in this area to identify the source of heavy metals.
Financial support and sponsorship
The authors thank the International Society of Nephrology (Clinical Research Grant #05-038), Brussels, Belgium, for the financial grant for the project.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors thank the management, Nanjappa Hospital, Shivamogga, Karnataka, India, and the staff of Nanjappa Hospital and Nanjappa Institute of Nursing Sciences for logistic support.
References
- Chronic kidney disease: Global dimension and perspectives. Lancet. 2013;382:260-72.
- [Google Scholar]
- Global prevalence of chronic kidney disease – A systematic review and meta-analysis. PLoS ONE. 2016;11:e0158765. doi: 10.1371/journal.pone. 0158765
- [Google Scholar]
- The burden of non communicable diseases in developing countries. Int J Equity Healt. 2005;4:2.
- [Google Scholar]
- The impact of CKD identification in large countries: The burden of illness. Nephrol Dial Transplant. 2012;27(Suppl 3):iii32-8.
- [Google Scholar]
- Chronic kidney disease (CKD) in disadvantaged populations. Clin Kidney J. 2015;8:3-6.
- [Google Scholar]
- CKD of unknown origin in Central America: The case for a Mesoamerican nephropathy. Am J Kidney Dis. 2014;63:506-20.
- [Google Scholar]
- Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: Geographical distribution and environmental implications. Environ Geochem Health. 2011;33:267-78.
- [Google Scholar]
- Clinical and pathological characterization of Mesoamerican nephropathy: A new kidney disease in Central America. Am J Kidney Dis. 2013;62:908-18. doi: 10.1053/j.ajkd. 2013.05.019
- [Google Scholar]
- Tubulointerstitial damage as the major pathological lesion in endemic chronic kidney disease among farmers in North Central Province of Sri Lanka. Environ Health Prev Med. 2012;17:213-21.
- [Google Scholar]
- Chronic kidney disease of unknown etiology in agricultural communities. MEDICC Rev 2014:16.
- [Google Scholar]
- Uncertain etiologies of proteinuric-chronic kidney disease in rural Sri Lanka. Kidney Int. 2010;80:1212-21.
- [Google Scholar]
- Clinical characteristics of chronic kidney disease of nontraditional causes in salvadoran farming communities. MEDICC Rev. 2014;16:39-48.
- [Google Scholar]
- Mesoamerican nephropathy or global warming nephropathy? Blood Purif. 2016;41:135-8. doi: 10.1159/000441265
- [Google Scholar]
- Chronic kidney disease of uncertain aetiology: Prevalence and causative factors in a developing country. BMC Nephrol. 2013;14:180.
- [Google Scholar]
- Nephropathy/regional nephropathy in India: Preliminary findings and a plea for further research. Am J Kidney Dis. 2016;68:344-8.
- [Google Scholar]
- Prevalence of chronic kidney disease among adults in a rural community in South India: Results from the kidney disease screening (KIDS) project. Indian J Nephrol. 2014;24:214-21.
- [Google Scholar]
- Hypertension is an important risk determinant for chronic kidney disease: Results from a cross-sectional, observational study from a rural population in South India. J Hum Hypertens. 2017;31:327-32.
- [Google Scholar]
- OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. Available from: www.OpenEpi.com
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Chapter 1: Definition and classification of CKD. Kidney Int Suppl. 2013;3:19-62. doi: 10.1038/kisup. 2012.64
- [Google Scholar]
- Testing for chronic kidney disease: A position statement from the National Kidney Foundation. Am J Kidney Dis. 2007;50:169-80.
- [Google Scholar]
- The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-72.
- [Google Scholar]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33(Suppl 1):S62-9.
- [Google Scholar]
- World Health Organization Western Pacific Region, International Association for the Study of Obesity, International Obesity Task Force. Redefining Obesity and Its Treatment 2000:1-56. ISBN: 0-9577082-1-1
- [Google Scholar]
- Urinary β2-microglobulin is a good indicator of proximal tubule injury: A correlative study with renal biopsies. J Biomarkers 2014 Article ID 492838; 2014. doi: 10.1155/2014/492838
- [Google Scholar]
- Mayo Clinic, Test Clinical and Interpretive. Available from: http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/60246
- Low molecular weight proteinuria in Chinese herbs nephropathy. Kidney Int. 1995;48:1571-6.
- [Google Scholar]
- β2-microglobulin: Its significance in the evaluation of renal function. Kidney Int. 1987;32:635-41.
- [Google Scholar]
- Possible link of chronic arsenic toxicity with chronic kidney disease of unknown etiology in Sri Lanka. J Nat Sci Res. 2013;3:64-73.
- [Google Scholar]
- Chronic kidney disease of unknown aetiology in Sri Lanka: Is cadmium a likely cause? BMC Nephrol. 2011;12:32. doi: 10.1186/1471-2369-12-32
- [Google Scholar]
- Glyphosate, hard water and nephrotoxic metals: Are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka? Int J Environ Res Public Health. 2014;11:2125-47.
- [Google Scholar]
- Variation in rice cadmium related to human exposure. Environ Sci Technol. 2013;47:5613-8.
- [Google Scholar]
- Drinking well water and occupational exposure to Herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environ Health. 2015;14:6.
- [Google Scholar]
- Chronic renal failure among farm families in cascade irrigation systems in Sri Lanka associated with elevated dietary cadmium levels in rice and freshwater fish (Tilapia) Environ Geochem Health. 2008;30:465-78.
- [Google Scholar]
- Climate change and the emergent epidemic of CKD from heat stress in rural communities: The case for heat stress nephropathy. Clin J Am Soc Nephrol. 2016;11:1472-83.
- [Google Scholar]
- Chronic kidney disease in two coastal districts of Andhra Pradesh, India: Role of drinking water. Environ Geochem Health. 2013;35:439-54.
- [Google Scholar]
- Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol. 2015;16:103. doi: 10.1186/s12882-015-0109-2
- [Google Scholar]
- Mechanisms of nephrotoxicity from metal combinations: A review. Drug Chem Toxicol. 2000;23:1-12.
- [Google Scholar]
- Association of CYP1A1 gene polymorphism with chronic kidney disease: A case control study. Environ Toxicol Pharmacol. 2013;36:164-70.
- [Google Scholar]







