Translate this page into:
Hemophagocytic Syndrome with Bone Marrow Tuberculosis in Renal Transplant Recipient
Address for correspondence: Dr. Sukanto K. Das, Department of Nephrology and Renal Transplant, AMRI Hospitals, Bhubaneswar, Odisha, India. E-mail: drsukantdas@yahoo.co.in
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Dear Editor,
Hemophagocytic syndrome (HPS) or macrophage activation syndrome is caused due to the systemic proliferation of hemophagocytic cells which belong to the monocyte-macrophage-histiocyte series. Apart from the familial variant of HPS which is due to a genetic defect seen in children, the secondary or reactive type can develop at any age. Immunocompromised patients are more vulnerable due to the presence of opportunistic infections which trigger HPS. Limited cases of HPS in renal transplant recipients have been reported and our case is one such illustration.
A 55-year-old male post-renal transplant recipient was hospitalized for fever, multiple episodes of loose motion, and generalized weakness in June 2020. He was a known case of diabetes and hypertension for five years before transplantation and had been on dialysis for six months before undergoing a living-donor renal transplant, with his wife being the donor, in the year 2015. He had received induction with basiliximab and methylprednisolone, and was maintained on tacrolimus, prednisolone, and mycophenolate mofetil (MMF). There had been no surgical complications or any episodes of acute rejection after the transplant.
On admission, his Hb was 8.2 gm/dl, TLC was 3100/cubic mm, and serum creatinine was 1.0. On the next day, TLC reduced to 800/cubic mm and the MMF was stopped temporarily, and the patient was started on broad-spectrum IV antibiotics, with IV fluids, and continued on modified immunosuppressive drugs with prednisolone and tacrolimus. Cytomegalovirus (CMV) PCR was negative.
The bone marrow aspirate smear showed features of hemophagocytic activity showing lymphocytes and RBSC engulfed by macrophage [Figure 1a]. The bone marrow study was suggestive of hypoplastic marrow with necrotizing granulomatous change and positive AFB, indicative of tubercular etiology. Bone marrow trephine biopsy showed caseating granuloma [Figure 1b], epithelioid cell granuloma [Figure 1c] along with necrosis [Figure 1d]. On special staining for acid-fast bacteria (AFB), bone marrow revealed positive staining [Figure 2].

- a) Bone marrow aspirate smear with features of hemophagocytosis showing lymphocytes and RBCs engulfed by macrophage (Giemsa, X 400); b) bone marrow trephine biopsy showing caseating granuloma (H and E, X 200); c) bone marrow trephine biopsy showing epithelioid cell granuloma (H and E, X 400); and d) bone marrow trephine biopsy showing necrosis with marrow elements (H and E, X 400).
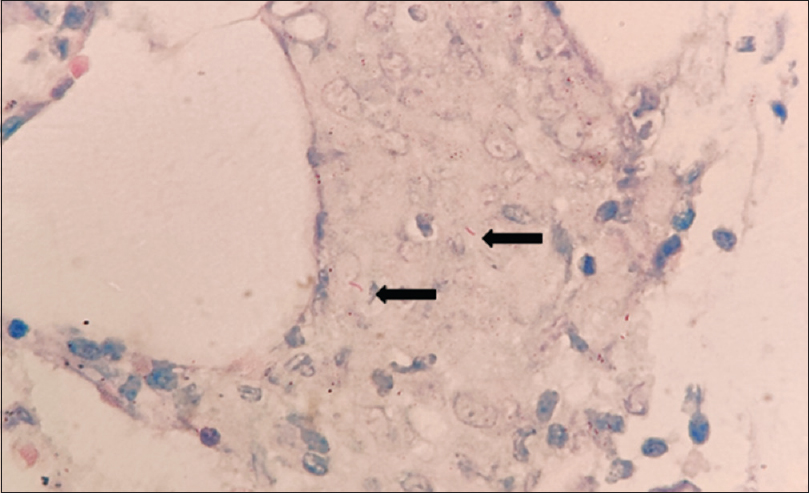
- Bone marrow trephine biopsy reveals positive staining for acid-fast bacilli as indicated by black arrows (Ziehl-Neelsen stain, x400)
Therefore, the patient was started on anti-tubercular treatment (ATT). For persistent leukopenia, the patient was treated with filgrastim. ATT consisting of four drug regimens with RIPE was administered. On improvement in TLC, MMF was reintroduced to the maintenance immunosuppression regimen. Appropriate dose adjustment of tacrolimus was done. The patient was clinically stable upon discharge. On subsequent follow-up, clinical improvement was observed. The patient was subsequently followed up over tele-medicine, and he was continued on rifampicin and isoniazid after two months and was doing well till February 2021. Subsequently, telephonic enquiry revealed that he died of cardiac arrest and we could not obtain any records related to his terminal events.
Reactive HPS is a rare syndrome seen in transplant recipients that is observed in systemic infections triggered by opportunistic infections like our case due to TB. CMV is common in transplant recipients, hampering the immune response and thereby favouring or activating the latent TB infection, which in rare instances develops into reactive HPS. HPS is seen in all age groups with the familial variant seen since childhood. The secondary or reactive HPS is seen in association with certain infectious disease, malignancies, or connective tissue diseases.[1] It is also reported in patients who are immunocompromised such as solid organ transplant recipients. It can cause severe mortality, multiple organ failure, or sepsis. As symptoms vary, it may be difficult to clinically diagnose and may increase the risk of it worsening. In a case series by Karras et al.,[2] the outcome of HPS in renal transplant patients was poor, with 47% mortality and 44.4% loss of graft; two cases of TB were seen. In another retrospective study by Emmanuel C, et al.[3] mortality rate was 60%, and 10.2% of cases had HPS along with TB.
Clinical manifestation of TB in transplant recipients are varied thereby delaying early detection and management. The majority of cases develop TB symptoms in the first-year post transplantation, and with newer immunosuppressive regimes such tacrolimus or mycophenolate, its development is seen earlier.[4] Our findings were contradictory as the patient developed complaints after five years since renal transplant. Symptoms of fever, organomegaly (liver or spleen), pancytopenia, increased triglycerides and ferritin levels were seen. Though increased ferritin, triglycerides, and LDH raise suspicion, bone marrow studies are the most sensitive tests to confirm the diagnosis of HPS.[5]
In the present case, development of HPS was late (five years). The clinical presentation was vague and extra-pulmonary manifestations were seen. Pancytopenia with fever posed a suspicion which with the help of bone marrow study was confirmed. Required management along with ATT yielded desirable outcome.
In conclusion, HPS in association with TB in post-transplant recipients is a rare finding, which must be considered in case of fever, organomegaly, and pancytopenia. Bone marrow study is the most sensitive test to confirm the diagnosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Tuberculosis-associated haemophagocytic syndrome. Lancet Infect Dis. 2006;6:447-54.
- [Google Scholar]
- Hemophagocytic syndrome in renal transplant recipients: Report of 17 cases and review of literature. Transplantation. 2004;77:238-43.
- [Google Scholar]
- Tuberculosis following kidney transplantation: Clinical features and outcome. A French multicentre experience in the last 20 years. Nephrol Dial Transplant. 2011;26:3773-8.
- [Google Scholar]
- Tuberculosis in renal transplant recipients on various immunosuppressive regimens. Nephrol Dial Transplant. 2005;20:797-802.
- [Google Scholar]
- Familial and acquired hemophagocytic lymphohistiocytosis. Eur J Pediatr. 2007;166:95-109.
- [Google Scholar]






