Translate this page into:
Herpes Simplex Virus Type-2 Nephritis: An Unexpected Plot Twist in a Kidney Transplant Recipient
Corresponding author: Dr. Lovy Gaur, Department of Nephrology and Kidney Transplant, Max Superspeciality Hospital, Sector- 1, Vaishali, Ghaziabad - 201 002, UP, India. E-mail: drlovygaur@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gaur L, Duggal R, Singhal MK. Herpes Simplex Virus Type-2 Nephritis: An Unexpected Plot Twist in a Kidney Transplant Recipient. Indian J Nephrol. 2024;34:512-3. doi: 10.4103/ijn_359_23
Abstract
Infections remain an important cause of morbidity in kidney transplant recipients, particularly in the early post-transplant period. This window coincides with an increased risk of acute rejections. Prompt identification of the cause of graft dysfunction is paramount to ensure good outcomes. This case report presents a 32-year-old male undergoing his second living-related kidney transplantation, complicated by herpes simplex virus-2 (HSV-2) nephritis. Despite favorable initial graft function, he developed odynophagia post-operatively, leading to the diagnosis of HSV-related esophageal ulcers. Subsequent acute graft dysfunction prompted biopsy, revealing HSV-2-related acute tubular injury. Prompt initiation of intravenous acyclovir resulted in graft recovery. This case underscores the importance of considering uncommon viral etiologies in post-transplant complications and highlights the role of timely diagnosis and treatment in preserving graft function.
Keywords
Herpes simplex virus-2 Nephritis
HSV nephritis
Post-transplant viral infections
Graft dysfunction
Kidney transplantation
Acute rejection
Introduction
Infections frequently complicate the course of solid organ transplant recipients. Herpes simplex virus (HSV) infection can be acquired from the environment, and the disease may result from the reactivation of latent infection or transmission from the donor. Although graft dysfunction caused by the direct impact of HSV, such as interstitial nephritis, is recognized, only a few cases have histopathologically confirmed the presence of HSV. In this report, we present the clinical course of a kidney transplant recipient who developed HSV-related nephritis and subsequently achieved complete recovery following antiviral therapy.
Case Report
A 32-year-old gentleman was admitted for a second living-related kidney transplantation from his mother. He underwent fulguration of posterior urethral valves at the age of 10 years but gradually progressed to end-stage kidney disease over the next decade. He had undergone his first kidney transplantation in 2010 with his father as the donor. However, his creatinine levels increased to 1.5 mg/dL the following year, prompting a graft biopsy, which revealed features of chronic allograft nephropathy. The graft failed over the next decade, necessitating the evaluation for a pre-emptive kidney transplantation.
He had a 8/12 HLA-allelic match and had low panel reactive antibody (Class 1%–4% and Class 2%–7%). Both the donor and the recipient were seropositive for cytomegalovirus (CMV) antibodies. He received 5 mg/kg antithymocyte globulin for induction and was initiated on triple-drug immunosuppressive therapy with tacrolimus, mycophenolate sodium, and prednisolone. The patient achieved good graft function, with a decline in serum creatinine to 0.7 mg/dL by postoperative day 3.
However, on postoperative day 6, the patient developed odynophagia, prompting an upper GI endoscopy, which revealed esophageal ulcers, and the histopathology findings were suggestive of HSV-related ulcers [Figure 1]. Valganciclovir dosage was increased to 900 mg/day. His symptoms abated, and he was discharged after 2 days, with a serum creatinine level of 0.9 mg/dL.

- Esophageal ulcer shows keratinocytes with viral cytopathic changes in the form of nuclear enlargement with ground glass appearance (black arrow) (original magnification ×400; Hematoxylin and Eosin). HSV2 immunohistochemistry shows nuclear positivity in the affected keratinocytes (black arrow) (original magnification × 400; HSV2 immunohistochemistry).
During a routine outpatient visit on postoperative day 14, the patient’s serum creatinine had risen to 2.1 mg/dL and tacrolimus level was 3.92 ng/mL. Systemic examinations, including genitals, were unremarkable. Suspecting acute rejection, a kidney biopsy was performed and the patient was pulsed with 250 mg methylprednisolone per day for 3 days. Biopsy revealed diffuse acute tubular injury with cytopathic changes in tubular cells [Figure 2]. Immunohistochemistry showed staining for HSV-2 in some tubular nuclei, consistent with HSV nephritis. Intravenous acyclovir was initiated at a dose of 10 mg/kg thrice daily. Subsequently, the graft function improved, and the serum creatinine decreased to 0.9 mg/dL over the next 2 days. Acyclovir was continued for a total of 2 weeks. This was followed by valganciclovir prophylaxis for the next 3 months.
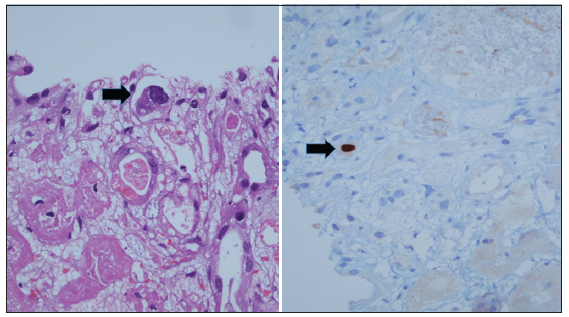
- Renal biopsy shows patchy cortical infarction with occasional large endothelial cells (black arrow) with ground glass nuclei (original magnification ×400; Hematoxylin and Eosin). HSV2 immunohistochemistry shows nuclear positivity (black arrow) in the enlarged endothelial cell (original magnification ×400; HSV2 immunohistochemistry).
Discussion
Approximately 70% kidney transplant recipients experience at least one episode of infection within 3 years. The risk of infection primarily depends on the exposure to potential microorganisms and the host’s susceptibility, which is influenced by net immunosuppression status.
In India, the seroprevalence of HSV-2 in the adult population is 10.1%.1 Post-transplant HSV infections are, however, uncommon, probably because the anti-cytomegaloviral prophylaxis with valganciclovir is also protective against HSV.
HSV infection is typically acquired through abraded skin or mucosal surfaces. After primary infection, the virus can remain dormant in ganglionic neurons and may be reactivated in states of immunosuppression. Although instances of primary HSV infection transmission through transplantation are rare, they are well documented.2-4 Routine screening of potential donors and recipients for HSV serology is not performed at our center. However, the absence of any clinically apparent HSV illness in the donor makes this possibility less likely.
Only two reported cases of biopsy-proven HSV-related interstitial nephritis have been described in the literature. The first reported case was of a 35-year-old lady who developed HSV nephritis following a cadaveric kidney transplant. Graft function remained poor, leading to graft nephrectomy approximately 3 weeks post transplant.5 The second case-report described a patient who developed acute graft dysfunction eventually attributed to HSV-related acute hemorrhagic nephritis.6
In our patient, graft dysfunction was preceded by HSV-related esophageal ulcers. Although acute rejection was suspected clinically, graft biopsy played a crucial role in confirming the diagnosis. Timely recognition is essential as appropriate treatment leads to favorable graft recovery.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Prevalence and correlates of Herpes Simplex Virus-2 and syphilis infections in the general population in India. Sex Transpl Infect. 2011;87:94-100.
- [CrossRef] [PubMed] [Google Scholar]
- Transmission of fatal herpes simplex infection through renal transplantation. Transplantation. 1988;45:653-6.
- [CrossRef] [PubMed] [Google Scholar]
- Transmission of infection with herpes simplex virus by renal transplantation. J Infect Dis. 1987;155:202-6.
- [CrossRef] [PubMed] [Google Scholar]
- Herpes simplex virus-2 transmission following solid organ transplantation: Donor-derived infection and transplantation from prior organ recipients. Transpl Infect Dis. 2017;19:e12739.
- [CrossRef] [PubMed] [Google Scholar]
- Herpes simplex virus interstitial nephritis in a renal allograft. Clin Nephrol. 1990;33:264-8.
- [PubMed] [Google Scholar]
- Hemorrhagic herpes simplex virus type 1 nephritis: An unusual cause of acute allograft dysfunction. Am J Transpl. 2017;17:287-91.
- [CrossRef] [PubMed] [Google Scholar]







