Translate this page into:
High hemodialysis vascular access flow and impaired right ventricular function in chronic hemodialysis patients
Address for correspondence: Dr. S. Yilmaz, Dr. Ilhan Gürel Street, No. 9/4, Corum, Turkey. E-mail: drlabarna@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
There are limited data showing right ventricular preload increase due to high-flow arteriovenous fistulas (AVFs). This cross-sectional study investigated whether high AVF flow had an impact on right ventricular function in patients undergoing hemodialysis. Sixty-four patients aged between 18 and 85 years who were on routine hemodialysis with >2 hemodialysis sessions per week for at least 3 months via an AVF were studied. Patients with inadequate flow fistulas, severe chronic obstructive pulmonary disease, history of pulmonary embolism, primary pulmonary hypertension, severe mitral, aortic or pulmonary regurgitation, and/or stenosis were excluded. After an initial evaluation, 44 patients (mean age: 58.50 ± 16.84, male:female = 23:21) were considered eligible. Right ventricular function was assessed by tricuspid annular plane systolic excursion (TAPSE). AVF blood flow was measured with duplex ultrasound. There were 15 patients (34.1%) with a TAPSE of <16 mm. AVF blood flow was significantly higher in patients with impaired versus normal right ventricular function (1631.53 ± 738.17 vs. 1060.55 ± 539.92 min/ml, respectively, P = 0.003). Low left ventricular ejection fraction (odds ratio [OR]: 1.15, 95% confidence intervals [CI]: 1.007-1.334, P = 0.04), high interventricular septum thickness (OR: 1.64, 95% CI: 1.104–2.464, P = 0.01), and high AVF blood flow (OR: 1.00, 95% CI: 1.000-1.003, P = 0.03) were independent predictors of impaired right ventricular function. In addition to known risk factors that predominantly increase right ventricular afterload, excessive AVF blood flow was found to be independently associated with impaired right ventricular function, possibly by increasing right ventricular preload.
Keywords
Arteriovenous fistula
heart failure
tricuspid chronic renal failure
Introduction
Arteriovenous fistulas (AVFs) is the optimal vascular access for hemodialysis. The fistula first initiative created a trend toward the initial use of AVFs rather than the use of central venous catheters, and this trend resulted in approximately two-fold increase in AVF prevalence.[1]
Despite its high success rate for long-term hemodialysis access, AVFs tend to be complicated by flow derangements. The long-term patency of a native AVF has been of concern since patency was linked to patient survival.[2] Stenosis or occlusion are major problems that have increasingly been treated by percutaneous catheter-based interventions.[3] On the other hand, high access flows may cause diffuse aneurysmal degeneration, steal syndrome, hand ischemia or cardiac overload.[45]
Chronic heart failure can develop due to volume overload between dialysis sessions, sympathetic discharge, ventricular hypertrophy, anemia, arterial stiffness, and vascular access flow. Each one of these mechanisms may partly contribute to an increase in either preload or afterload of both ventricles in the given patient.[6] There are l Right ventricular preload may be caused by high-flow AVF; however, right ventricular dysfunction and secondary pulmonary hypertension have not directly been linked to the presence of a high-flow access.[78] The present study has focused on whether high AVF flow has an impact on right ventricular function in patients receiving hemodialysis.
Patients and Methods
The cross-sectional study was approved by local ethics committee, and informed consent was obtained from patients. Patients aged between 18 and 85 years, undergoing >2 hemodialysis sessions per week for at least 3 months via an AVF were studied in Nov-Dec 2014. Patients with AVF with inadequate flow (<600 ml/min), severe chronic obstructive pulmonary disease, history of pulmonary embolism, primary pulmonary hypertension, unstable angina pectoris, recent myocardial infarction (<1 month), history of heart valve surgery, severe mitral, aortic or pulmonary regurgitation, and/or stenosis were excluded. Among a total of 64 consecutive patients, 44 (mean age: 58.50 ± 16.84, male:female = 23:21) were considered eligible and were included in the study.
Patient counseling and physical examination were performed by cardiovascular surgeons. All patients underwent a transthoracic echocardiography (TTE) which was performed by two senior cardiologists on the day after a planned hemodialysis session to obtain euvolemic status. Transthoracic two-dimensional and Doppler echocardiographic examination were performed with a Vivid S5 Cardiovascular Ultrasound machine (GE® Medical Systems, Phoenix, USA) using a 2.0 MHz transducer. Examinations were made with the patient placed in left lateral decubitus position. Left ventricular end-systolic diameters (LVESD) and left ventricular end-diastolic diameter, left atrial diameter, and interventricular and posterior wall thickness were measured in apical two or four-chamber views. Right ventricular systolic function was assessed based on tricuspid annular plane systolic excursion (TAPSE) measured by M-mode echocardiography. To avoid measuring TAPSE incorrectly due to angle dependency, the cursor was set parallel to the tricuspid annulus. Duplex examinations were performed on the same day of TTE examination by a senior radiologist. Using color duplex scanner Logiq p6 (GE® Healthcare, USA) with 9 MHz transducer, longitudinal and transverse B-mode and color flow images were obtained to calculate AVF blood flow distal to the arterial anastomosis site.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. Categorical variables were compared using Chi-square test and Fisher's exact test where appropriate. Continuous variables with normal distribution were compared using independent samples t-test. Nonparametric data were compared using Mann–Whitney U-test. Parameters showing significant differences between patients with and without impaired right ventricular systolic dysfunction were further evaluated by multivariate binominal logistic regression analysis. Appropriateness of the model was tested using Akaike information criterion. P ≤ 0.05 was considered as statistically significant.
Results
Baseline characteristics were compared between patients with a TAPSE ≥16 mm and <16 mm [Table 1]. There were no significant differences between two group of patients with regard to clinical and laboratory parameters.

There were no patients with New York Heart Association (NYHA) Class IV symptoms. There were a total of 15 patients (34.09%) with a TAPSE <16 mm. Of these, four patients had NYHA Class III symptoms and the remaining did not have heart failure symptoms. Left ventricular ejection fraction (LVEF) was significantly lower in patients with TAPSE <16 mm compared to those with TAPSE ≥16 mm (45.66 ± 4.57% vs. 52.75 ± 8.72%, P = 0.006). LVESD (37.80 ± 6.21 mm vs. 33.75 ± 5.29 mm, P = 0.002), posterior wall thickness (15.13 ± 2.23 mm vs. 13.62 ± 2.38 mm, P = 0.02) and interventricular septum thickness (16.06 ± 2.43 mm vs. 13.51 ± 2.08 mm, P = 0.002) were significantly higher in patients with TAPSE <16 mm than those with TAPSE ≥16 mm. AVF flow was significantly higher in patients with TAPSE <16 mm than those with TAPSE ≥16 mm (1631.53 ± 738.17 vs. 1060.55 ± 539.92, P = 0.003) [Figure 1]. Although a brachiocephalic AVF was more common than a radiocephalic AVF in TAPSE <16 mm group, the difference was not of statistical significance (11 [73.3%] vs. 16 [55.2%], P = 0.24).
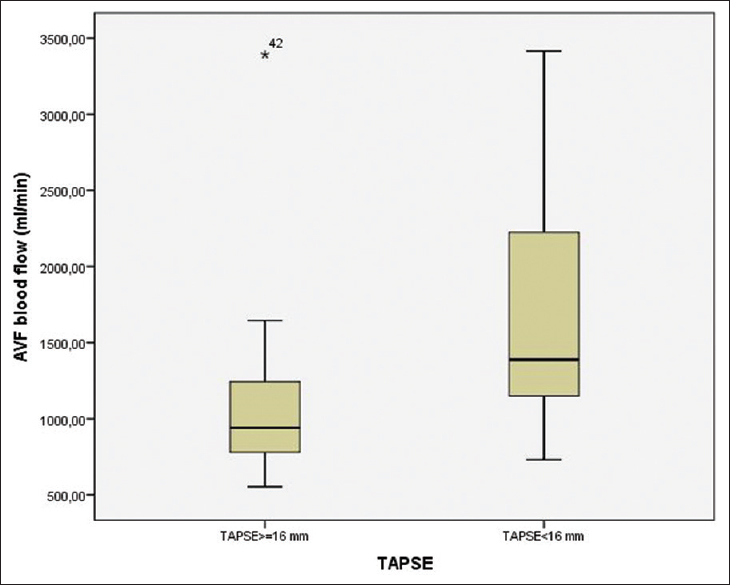
- Box-plot for arteriovenous fistulas blood flow between patients with and without right ventricular dysfunction
In multivariate analysis, low LVEF (odds ratio [OR]: 1.15, 95% confidence intervals [CI]: 1.007-1.334, P = 0.04), high interventricular septum thickness (OR: 1.64, 95% CI: 1.104-2.464, P = 0.01), and high AVF blood flow (OR: 1.00, 95% CI: 1.000-1.003, P = 0.03) were independent predictors of impaired right ventricular function [Table 2].

Discussion
Our study demonstrated that high blood flow via a functioning AVF may contribute to right ventricle (RV) systolic dysfunction that is more common than recognized in patients with ESRD. However, RV functional impairment cannot not be solely attributed to the increased preload to the right side of the heart through the high arteriovenous return since LVEF and wall thickness were also emerged as independent predictors of impaired RV systolic function in multivariate analysis.
The prevalence of pulmonary hypertension in hemodialysis patients ranges from 20% to 41%, but the prevalence of right ventricular dysfunction has rarely been investigated in this population. In a recent cross-sectional study,[9] echocardiographic examination revealed that 32 of 70 patients (45.71%) on routine hemodialysis had right ventricular dysfunction, which was higher than we have found (34.09%) in the present study. However, criteria for diagnosis of right ventricular dysfunction is unequivocal in that study where TAPSE values were somewhat higher (about >20 mm) than the threshold suggested by current guidelines (<16 mm).[10]
On the other hand, our results are parallel to some previous studies. Paneni et al.[11] performed an echocardiography study in 220 subjects, of which 94 patients had an AVF for hemodialysis access. The authors reported that tissue Doppler imaging indices of RV function were found to be significantly decreased in hemodialysis patients, particularly in those with a brachial AVF rather than radial AVF. However, AVF blood flow measurements were not taken into consideration in this study.
In another study, Di Lullo et al.[12] included 20 patients with ESRD and evaluated the effect of single hemodialysis on right ventricular function. In this study, half of the patients had radiocephalic AVF whereas the remaining had central venous catheters. The authors demonstrated that patients with AVF had greater RV diameters and lower TAPSE values compared to those with central venous catheters. The authors suggested that lower TAPSE values were due to increased preload that was produced by AVF, although no data were given regarding the flow measurements.
Clarkson et al.[7] reported a 52-year-old man with ESRD in whom they successfully treated secondary pulmonary hypertension by ligation of a high-flow AVF. In their report, duplex confirmed blood flow through the AVF was 3.6 L/min, and they found that oxygen saturations were elevated in superior vena cava, right atrium, and RV. The authors noted that pulmonary artery systolic pressure decreased from 68 mmHg to 30 mmHg, 5 days after ligation of the fistula.
Systemic effects of a high-flow AVF were also addressed in a recent study.[13] In this study, 34 patients underwent surgical intervention for high-flow AVF and a temporary clamping (15 s) produced a 12 ± 2 mmHg increase in systolic blood pressure and 6 ± 1 mmHg increase in diastolic blood pressure. The study also demonstrated that cardiovascular overload progressively increased with higher access flows >2–2.5 L/min.
Cardiovascular disease accounts for more than 50% of deaths in patients with ESRD. Although acute myocardial infarction is the most common mode of death, left ventricular hypertrophy and dilatation are also well-known predictors of cardiac mortality in patients receiving long-term renal replacement therapy.[14] Patients on chronic hemodialysis are faced with a substantial increase in left ventricular mass that is due to chronic overflow resulting from anemia and return from AVF, even though they do not have any cardiovascular disease.[15]
Although changes in left ventricle function and structure have been well-established, less is known regarding right ventricular impairment and its impact on survival in ESRD patients. In a retrospective study, Stallworthy et al.[16] reviewed echocardiographic data of patients in renal transplantation waiting list and reported that pulmonary hypertension and/or right ventricular function was associated with an increased risk of all-cause mortality. Left ventricular dysfunction and regional wall motion abnormalities were among the independent predictors of survival in this study.
Dini et al.[8] performed a study on 373 heart failure patients with LVEF <45% and found that TAPSE ≤14 significantly increased the odds of lower glomerular filtration rate (GFR), concluding that right ventricular dysfunction was associated with chronic kidney disease and prospectively predicted survival among this population. These authors reported that mortality was lowest in patients with TAPSE >14 mm and estimated GFR >60 ml/min/1.73 m2. However, this study was not focused on the effects of AVF flow on right ventricular performance.
Effect of sodium and water retention should also be noted in the development of right ventricular dysfunction in patients with ESRD, and therefore our results should cautiously be interpreted. ESRD patients with volume overload have increased levels of vasopressin, increased renin-angiotensin-aldosterone activity and sympathetic activity that contribute to right ventricular impairment regardless of the high venous return from a high-flow AVF. However, as mentioned above, these well-known mechanisms for left ventricular failure are yet to be clarified for RV failure in ESRD.[17]
Limitations
A cross-sectional design was the major limitation of the present study and we could not provide follow-up data in terms of survival and morbidity. Small sample size was another limitation, and our results await confirmation by further study on large populations. Although both TTE and Doppler flow measurements were performed on the same day (the day after the hemodialysis session), effect of volume loading may have affect the measurements in some patients. In addition, although patients who might potentially have right ventricular dysfunction due to certain diseases including pulmonary disease or valvular heart disease were not included, patients with chronic ischemic heart disease were not excluded from the study unless they had unstable angina pectoris or recent myocardial infarction. We are aware that this was one of the important limitations of the present study, which would be reconsidered in future studies.
Conclusion
In addition to known risk factors that predominantly increase right ventricular afterload, excessive AVF blood was found to be independently associated with impaired right ventricular function, possibly by increasing right ventricular preload. Our study suggests that AVF blood flow should necessarily be addressed as a potential contributor to impaired right ventricular function in further studies aiming to reveal its pathogenesis and treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Fistula first breakthrough initiative: Targeting catheter last in fistula first. Semin Dial. 2012;25:303-10.
- [Google Scholar]
- Primary patency rates of AV fistulas and the effect of patient variables. J Vasc Access. 2008;9:45-50.
- [Google Scholar]
- Why vascular access trials on flow surveillance failed. J Vasc Access. 2014;15(Suppl 7):S15-9.
- [Google Scholar]
- Banding of hemodialysis access to treat hand ischemia or cardiac overload. Semin Dial. 2009;22:204-8.
- [Google Scholar]
- Diffuse aneurysmal degeneration of the brachial artery after long-standing high-flow arteriovenous fistula closure for hemodialysis at elbow level. Ann Vasc Surg. 2014;28:1315.e11-5.
- [Google Scholar]
- Mechanisms of chronic heart failure development in end-stage renal disease patients on chronic hemodialysis. Physiol Res. 2009;58:613-21.
- [Google Scholar]
- Reversal of pulmonary hypertension after ligation of a brachiocephalic arteriovenous fistula. Am J Kidney Dis. 2002;40:E8.
- [Google Scholar]
- Right ventricular dysfunction is associated with chronic kidney disease and predicts survival in patients with chronic systolic heart failure. Eur J Heart Fail. 2012;14:287-94.
- [Google Scholar]
- Pulmonary hypertension and right ventricular dysfunction in hemodialysis patients. Eur Rev Med Pharmacol Sci. 2014;18:3267-73.
- [Google Scholar]
- Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685-713.
- [Google Scholar]
- Right ventricular dysfunction in patients with end-stage renal disease. Am J Nephrol. 2010;32:432-8.
- [Google Scholar]
- Right ventricular diastolic function in dialysis patients could be affected by vascular access. Nephron Clin Pract. 2011;118:c257-61.
- [Google Scholar]
- Systemic effects of a high-flow arteriovenous fistula for hemodialysis. J Vasc Access. 2014;15:163-8.
- [Google Scholar]
- Contributive factors to cardiovascular hypertrophy in renal failure. Am J Hypertens. 1989;2(11 Pt 2):261S-3S.
- [Google Scholar]
- Do echocardiographic parameters predict mortality in patients with end-stage renal disease? Transplantation. 2013;95:1225-32.
- [Google Scholar]
- Pulmonary hypertension, right ventricular failure, and kidney: Different from left ventricular failure? Clin J Am Soc Nephrol. 2008;3:1232-7.
- [Google Scholar]







