Translate this page into:
Histopathological and Immunohistochemical Study of Acute Tubular Injury in Native Kidney Biopsy
Corresponding author: Swarnalata Gowrishankar, Department of Histopathology, Apollo Hospitals, Jubilee Hills, Hyderabad, Telangana, India. Email: swarnalatag@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mahajan V, Gowrishankar S. Histopathological and Immunohistochemical Study of Acute Tubular Injury in Native Kidney Biopsy. Indian J Nephrol. 2024;34:310-6. doi: 10.25259/ijn_282_23
Abstract
Background:
Acute tubular injury (ATI) is a common diagnosis on renal biopsy. There are no accepted parameters to assess the severity of injury or predict recovery. An objective histologic grading system would be of immense value in clinical practice. The macrophage response to injury involves the MI phenotype which is proinflammatory and M2 which is prorepair. The study of these macrophages could aid in studying the severity and the recovery.
Materials and Methods:
A total of 58 native kidney biopsies with features of ATI and a minimum follow-up of 12 weeks were graded into mild, moderate and severe, using scores for simplification, sloughing, and mitosis. These scores and the density of macrophages stained with CD68, CD163, and HLA-DR were correlated with serum creatinine at presentation and with recovery. The effect of chronicity index as measured by glomerulosclerosis, tubular atrophy, and interstitial fibrosis and of co-morbidities of age, hypertension, and diabetes on the recovery pattern was also studied.
Results:
All three histologic scores and the grades of ATI showed positive correlation with the serum creatinine level. The densities of CD 68 + and CD163 + macrophages also showed a significant correlation with serum creatinine level. However, none of these these histological features nor the macrophage densities predicted clinical recovery. Age >60 years, hypertension, diabetes, and chronicity score on biopsy were indicators of partial and delayed recovery.
Conclusion:
The histopathological semiquantitative scoring system can be used routinely to grade ATI. However none of the studied parameters predicted recovery.
Keywords
Acute tubular injury (ATI)
CD163
CD68
Histopathology
Macrophages

Introduction
The clinical syndrome of acute kidney injury (AKI) encompasses prerenal, intrinsic renal and post-renal causes with the categories based on dynamics of serum creatinine and urine output.1,2 AKI is a serious condition which not only affects the kidney function leading to significant morbidity and mortality, but also has long-term detrimental effects on kidney function which is determined by the cause, duration and severity of injury, associated comorbidities and renal reserve.3-5
Acute tubular injury (ATI) is likely to be responsible for majority of the cases of AKI requiring renal replacement therapy.6 The term ATI should be reserved for the clinicopathologic entity of intrinsic renal failure resulting from ischemic or toxic insult to the kidney with evidence of tubular injury and altered specific tubular injury markers.6
There are a multitude of tubular morphologic changes associated with ATI. There are very few studies correlating the histopathological features with clinical parameters. In one such study by Kudose et al.,7 a semiquantitative scoring system using features of tubular epithelial simplification, cell sloughing, and mitosis was developed. In the present study, we have used the same semiquantitative scoring system and correlated the scores and grades of tubular injury with serum creatinine, recovery status, and time to recovery. Comorbid conditions like age, hypertension, and diabetes, known to cause irreversible chronic injury to the kidney, were also correlated with the recovery profile.
Macrophages are highly heterogeneous and plastic inflammatory cells. When activated, they are polarized into different phenotypes (M1-proinflammatory and M2-pro repair) in response to injury. The polarized macrophages carry out specific functions by secretion of different cytokines and growth factors.8 The imbalance between M1 and M2 response or the persistence of M2 response is thought be to profibrotic.9,10 In this study, densities of the different types of macrophages, CD68 representing all macrophages, HLA-DR representing the M1 and CD163, the M2 phenotype, were correlated with serum creatinine, grades of injury, and the recovery status.
Materials and Methods
This is a prospective study conducted at Apollo Hospital, Hyderabad. A total of 58 native kidney biopsies, in the study period of 6 months from July 2021 to December 2021, in patients presenting with AKI and with pure histological features of ATI on histology were included. Biopsies with coexisting features of glomerulonephritis, overt interstitial nephritis, malignant hypertension/ thrombotic microangiopathy, etc., and biopsies showing background diabetic nephropathy were excluded.
Clinical and demographic data
Patient characteristics including age, gender, history of hypertension and diabetes, serum creatinine level at the time of biopsy (available in 55 cases), presence of hematuria and proteinuria were obtained. Follow-up after 12 weeks with serum creatine levels could be obtained in 46 cases and approximate time to recovery in cases of complete recovery was also noted. 12 patients were lost to follow-up. Complete recovery was defined as serum creatinine levels returning to normal range, while partial recovery was considered when the serum creatinine levels were reduced but did not return to normal range after a period of 12 weeks.5
Light microscopy
Each biopsy was studied with the help of Hematoxylin eosin, Periodic acid Schiff, Jones methenamine silver, and Masson trichrome stains. Three morphological features of ATI were evaluated and scored according to the definitions and scoring system proposed by Kudose et al. as described below.7 Total injury scores were calculated and cases were graded as mild/equivocal/severe depending on the total scores obtained in each biopsy [Figure 1].
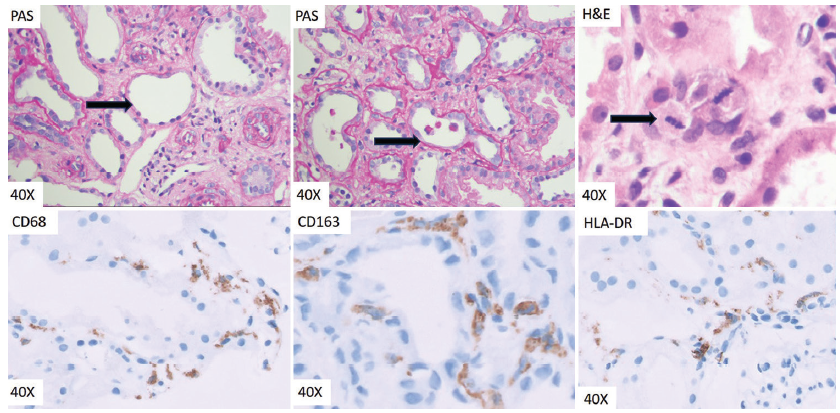
- Top left: PAS Arrow showing simplification of tubular epithelial cell with loss of brush borders, flattened lining and relatively dilated tubular lumina. Top middle: PAS -Arrow showing cell sloughing with detached tubular epithelial cells within the tubular lumina. Top right: H and E-Arrow showing two mitoses in a tubular epithelial cell. Bottom left: IHC for CD68 -Cytoplasmic CD68 positivity in interstitial macrophages. Bottom middle: IHC for CD163 -Cytoplasmic CD163 positivity in interstitial macrophages. Note the CD163 positive cells lying close to and surrounding the tubular basement membrane. Bottom right: IHC for HLA-DR -Microphotograph showing HLA-DR positive interstitial macrophage. PAS: Periodic acid-schiff; IHC: Immunohistochemistry; HLA: Human leucocyte antigen; H&E: Hematoxylin and eosin.
Scoring system to classify tubular injury
Simplification: Defined as tubular cross-section with flattened tubular cell cytoplasm (height unequivocally less than width), with complete loss of brush border involving >50% of the tubular cells in cross-section, resulting in an apparent increase in the size of lumen, without presence of casts. This was scored as 0 (0%), 1 (<25% tubules with simplification), 2 (25%–50% tubules with simplification), and 3 (>50% tubules with simplification).
Mitosis: Tubular epithelial cells in any mitotic phase, identified by distinctively visible chromosome in either prophase, metaphase, anaphase, or telophase configuration. The single 400 × field with the greatest number of tubular epithelial mitotic figures was scored. 0 (no mitosis), 1 (1 mitosis), 2 (2 mitoses), 3 (≥3 mitoses).
Cell sloughing: At least one free floating cell in the tubular lumen without attachment to adjacent cells or basement membrane in a tubular cross-section. These cells must not aggregate into a tubular shape and completely fill the lumen. The single 400 × field with the maximum number of tubules was scored. 0 (no sloughing), 1 (1 tubule with sloughing), 2 (2 tubules) 3 (≥3 tubules).
Acute tubular injury score
Total score = 2 × simplification score + 2 × mitosis score + cell sloughing score.
Grading of tubular injury
A score of ≤2 was graded mild, 3 as equivocal, and ≥4 as severe. The nomenclature proposed by Kudose et al. in their seminal article7 has been retained and hence the score of 3 was scored equivocal and not moderate.
Immunohistochemistry
Immunohistochemistry (IHC) was done on formalin-fixed paraffin-embedded tissue on the Ventana Benchmark XT platform (n = 57). CD 68 (prediluted anti CD68 -KP-1 mouse monoclonal antibody PathInSitu-Cat# HAM 113), CD163 (prediluted Tinto anti-CD 163 mouse monoclonal antibody, Bio SB, clone 10D6) HLA-DR (prediluted Tinto anti HLA-DR α chain Bio SB clone EP 96) were employed.
The total number of interstitial cells with cytoplasmic positivity for CD 68/CD163/HLA-DR were counted, across the complete length of the cortex in each case. The density was calculated with the exact area of the cortex obtained by a whole slide digital image on the Philips IntelliScan system using the Philips Image Management Software (Philips Healthcare, Andover, MA 01810-1099, United States of America) [Figure 1].
Statistical analysis
Descriptive statistics was presented in the form of proportions/percentages for categorical variables and mean and standard deviation for continuous variables. Fisher’s exact test/Chi square test was used for the comparison of categorical variables while continuous variables were analyzed using Mann Whitney test/Student’s t test (for unpaired data) and Wilcoxon sign rank test/paired t test (for paired data) based on the normality of the data. Multiple groups were analyzed using Anova/ Kruskal Wallis test with post hoc analysis. A receiver operator curve analysis was also performed using standard parameters. A P value of < 0.05 was considered significant. The data were compiled and analyzed using MS excel (R) office 365, GraphPad prism 8.4.2, and SPSS version 25.
Results
Baseline demographic and clinical characteristics and the recovery profile of the patients are shown in Table 1. Only 6 cases were under the age of 6 years.
| Demographic and clinical profile (n = 58) | |
|---|---|
| Age (years) [Mean ± SD] | 44.43 ± 20.27; Range-1-85 |
| Sex (%) | 29.31%-Female; 70.69%-Male |
| Serum Creatinine (mg/dl) [Mean ± SD] | 6.37±3.80; Range-1.17-19 |
| Hypertension (%) | 44.82% |
| Diabetes (%) | 10.34% |
| Any hematuria | 44.82% |
| Any proteinuria | 67.24% |
| Recovery profile (n 46, 12 patients-lost to follow up) | |
| Complete recovery | 28 (60.86%) |
| Partial recovery | 18 (39.13%) |
| Time to complete recovery (weeks) [Mean ± SD] | 3.86 ± 1.84; Range 1-8 |
Pathological characteristics in different grades of ATI
The histopathological scores, grades of tubular injury, scores and grades of chronicity are shown in Table 2.
| Tubular injury score | P | Significance | |
|---|---|---|---|
| Simplification score [Mean ± SD] | 1.38 ± 0.85; Range 0-3 | 0.0004 | Yes |
| Mitosis score [Mean ± SD] | 0.60 ± 0.75; Range 0-3 | 0.0326 | Yes |
| Cell sloughing score [Mean ± SD] | 1.10 ± 0.93; Range 0-3 | 0.0376 | Yes |
| Total score | 5.05 ± 3.07; Range 1-13 | 0.0002 | Yes |
| Grade of injury | |||
| Mild (n = 10) | Equivocal vs mild | 0.2566 | No |
| Equivocal (n = 17) | Mild vs severe | <0.0001 | Yes |
| Severe (n = 31) | Equivocal vs severe | 0.0068 | Yes |
More than half of the cases (53.44%) had severe grade of tubular injury, while 29.31% had equivocal, and 17.24% had mild tubular injury on histological examination.
Serum creatinine level was found to have positive correlation with all three histological parameters of ATI studied, with significant P value. The calculated total injury score also showed positive correlation with the serum creatinine. There was significant difference in serum creatinine levels when compared between cases of mild vs severe and equivocal vs severe injury. The serum creatinine levels were the highest with severe grade of injury [Table 2].
Proteinuria, as assessed by the dipstick was positive in 39 of 44 patients, but the intensity did not have any relation to the histologic grade of ATI.
There was no significant difference between values of simplification score, mitosis score, cell sloughing score, and total score when compared amongst patients with complete vs. partial recovery. However, it was found that as simplification score and total score increase, the time to recovery also increases (R > 0), but the correlation was not statistically significant. There was no difference in time to recovery amongst various grades of injury [Table 3].
| Histological parameter | Complete recovery (n = 28) Mean score | Partial recovery (n = 18) Mean score | P | Significance | Time to recovery (in weeks) | |
|---|---|---|---|---|---|---|
| P | Significance | |||||
| Simplification | 1.43 | 1.39 | 0.8795 | No | 0.1328 | No |
| Mitosis | 0.61 | 0.50 | 0.6192 | No | 0.5625 | No |
| Cell Sloughing | 0.96 | 1.06 | 0.7077 | No | 0.7833 | No |
| Total score | 5.00 | 4.83 | 0.8476 | No | 0.5272 | No |
| Chronicity parameters | ||||||
| Chronicity score (Mean) | 0.60 | 2.25 | 0.0019 | Yes | 0.2814 | No |
| Chronicity Grade | ||||||
| Nil | 68% | 31.25% | 0.0230 | Yes | – | – |
| Minimal | 16% | 18.75% | 0.8216 | No | – | – |
| Mild | 16% | 25% | 0.4835 | No | – | – |
| Moderate | 0 | 25% | 0.0093 | Yes | – | – |
| Comorbidities | ||||||
| Age >= 60 years | 14.28% | 44.44% | 0.0245 | Yes | 0.0012 | Yes |
| Hypertension | 46.42% | 55.55% | 0.5500 | No | 0.0251 | Yes |
| Diabetes | 7.14% | 16.67% | 0.3161 | No | 0.0815 | No |
On analysis of the etiology, it was predominantly ischemic in 12, toxic in 15, post-covid in 9, and not readily apparent in 17. The grade of injury was severe in 50% in the ischemic group and 86.6% in the toxic group. Complete recovery occurred in 70% in each of these two groups with a mean time of 4.2 weeks and 3.7 weeks, respectively [Table 4].
| Parameter | CD68/mm2 | CD163/mm2 | HLA-DR/mm2 |
|---|---|---|---|
| Number of positive cells/mm2 area (Mean) in MCD | 21.25 | 22.51 | 3.3 |
| Number of positive cells/mm2 area (n = 57) (Mean +/-SD) in ATI |
166.63 ± 132.14; Range 7.06-586.51 |
200.74 ± 136.35; Range 4.49-666.66 |
23.58 ± 26.25; Range 0.81-123.98 |
| Correlation with Serum creatinine | <0.0001 | 0.0006 | 0.0545 |
| (P) | (Statistically significant) | (Statistically significant) | (Not significant) |
| Correlation with the recovery status (complete vs partial) | |||
|
Complete recovery n = 28 (Mean number) |
189.77 | 217.23 | 21.61 |
|
Partial recovery n = 18 (Mean number) |
138.36 | 183.52 | 29.14 |
| P | 0.2030 | 0.3731 | 0.3969 |
| Significance | No | No | No |
| Correlation with the time to complete recovery (in weeks) | |||
| Pearson r | –0.12 | –0.062 | –0.14 |
| P | 0.5462 | 0.7543 | 0.4907 |
| Significance | No | No | No |
MCD: Minimal change disease; P: p value; ATI: Acute tubular injury, HLA-DR: Human leucyte antigen-DR.
Macrophage densities in ATI
The mean density of CD68, CD163, and HLA DR macrophages in 10 cases of minimal change disease (MCD) was taken as the baseline.
In contrast to MCD, the densities of macrophages positive for CD68, CD163, and HLA-DR were considerably higher in cases of ATI, with values of 166.13 ± 132.14, 200.74 ± 136.35, and 23.58 ± 26.25 respectively.
It was observed that both CD68+ macrophages and CD 163 + macrophages increase in number with increasing levels of serum creatinine with significant P value, being highest for the former. However, such positive correlation was not observed between serum creatinine levels and number of HLA-DR + macrophages. The densities of CD 68 macrophages correlated well with that of CD163 positive and HLA-DR positive macrophages with an R value of 0.87 and 0.63, respectively.
The patients with complete recovery had a slightly higher number of CD68+ and CD163+ macrophages when compared with the partial recovery group, however, this difference was not statistically significant. There was no correlation between number of HLA DR positive macrophages and the status of recovery.
A higher CD68 score was seen to be associated with shorter time for recovery (R < 0), but these results were not significant statistically.
Chronicity score
Around 58.82% patients had no chronicity on biopsy while 7.8% had moderate background chronicity, with chronicity score calculated according to that proposed by Sethi, et al.11 The chronicity score had an impact on recovery status as well as time for recovery. None of the patients with moderate chronicity recovered completely, while all the patients with no chronicity features had complete recovery from renal dysfunction. The chronicity score was significantly different amongst completely and partially recovered groups. [Table 3].
Chronicity score was positively associated with the time to recovery. This suggested that the time to recovery increased as the chronicity score increased, however, the results were not significant statistically [Table 3].
Age more than 60 years was found to be positively correlated with partial recovery. On similar trends, hypertension and diabetes were more commonly associated with partial recovery than complete, but the difference was not statistically significant [Table 3].
The mean time to recovery for the patients with age ≥60 years and hypertensive patients was significantly higher compared to the patients less than 60 years of age and nonhypertensive, respectively. Similarly, the mean time to recovery in the patients with diabetes was higher compared to the nondiabetes group, but the results were not significant statistically [Table 3].
Discussion
A systematic review of clinical characteristics and histologic descriptions of ATI by Wen et al. emphasized the diverse histological features seen in ATI and a need for standardizing pathology reporting to objectively quantify the severity of tubular injury, to establish the markers for renal recovery, to stimulate research, and eventually to search for therapeutic options.12 There are few studies which have tried to develop a scoring system based on the histological findings in ATI to look for clinical correlation and prognosis in native and transplant biopsies.7,13-15 However, these scoring systems have not been widely assessed in large cohorts of native kidney biopsies, and the prognostic value of each histologic description remains unclear.
In the present study, we found that the severity of tubular epithelial simplification, cell sloughing, and mitosis in tubular epithelial cells positively correlate independently with the serum creatinine level. Similarly, the grades of tubular injury calculated using the same parameters also correlate positively with the serum creatinine values.7 This result, if validated in larger studies, could lead to the clinician looking for other causes of a raised serum creatinine where the tubular injury scores are low. Moreover, in the present study only biopsies with pure ATI were included and those with glomerular, interstitial or other causes of AKI are excluded. The results, therefore, can be attributed to the component of tubular injury and the contribution by glomerular and other causes to ATI are considered nullified. In a study of histological features of ATI with delayed graft function (DGF) in transplant kidney biopsies, Pieters et al. evaluated morphological characteristics like edema, casts, vacuolization, and dilatation with a scoring system. They found features of edema and casts to significantly correlate with prognosis. However, whether these results hold true for native biopsies is uncertain. They also found number of Ki67 positive cells correlated with severity of tubular damage.14 On the similar ground, we also found the number of mitosis in tubular epithelial cells had a positive correlation with the severity of injury. Pizov and Friedlaender also concluded that proliferative activity in transplant kidney biopsies did correlate positively with the tubular damage.16
In literature, there has been no single histologic feature that could predict recovery from ATI. In our study too, there was no significant correlation between the three histological parameters studied as also the derived grades of ATI, with the recovery status of the patients or with the time to recovery. In a small study of native kidney biopsies of patients with acute renal failure (ARF), Abdulkader et al. analysed four histological features semi-quantitatively (tubular atrophy, interstitial inflammatory infiltrate, interstitial fibrosis, and acute tubular necrosis [ATN]). They also found that no single histological parameter was associated with partial recovery, but the sum of all four was when expressed as an injury index.13 Note that chronicity indices (IFTA) and inflammatory response were included in their scoring system. Pieters et al. also quantified regenerative potential of tubular cells using Ki67 and CD133 index, but none of them correlated with renal functional recovery. This was attributed to maladaptive regenerative response of kidney tubules.14
Recovery from ATI depends on multiple factors like severity and duration of insult, inflammatory response to injury, background chronicity, and the patient-related risk factors like high age and comorbidities such as hypertension and diabetes mellitus.5,17-20 Some of them indicate a reduced renal reserve. In consonance with these studies, our study also had an association of background chronicity, older age, hypertension, and diabetes with partial and delayed recovery from renal dysfunction.
With regard to the etiology, the numbers in each group were small with no single attributable cause in 17 cases. Besides, there was a significant overlap of the injury patterns in each group, and hence, further analysis etiology-wise was not pursued.
The limitation of the present study is that serum creatinine levels at the time of biopsy was considered and this may not be the best reflection of renal function. Biopsies are indicated in clinically severe AKI leading to skewed distribution of the studied cohort towards severe grade of ATI. Baseline creatinine and urine output were not known in all cases which are essential for staging clinical AKI. And lastly interobserver variability in scoring was not assessed.
Many studies have evaluated the role of macrophages in ATI and in progression to chronic kidney disease (CKD).8,10,21-24 Macrophages are known for their plasticity. In response to injury and dominant Th1 response (Interferon-IFN-γ), there is polarization of macrophages to M1 type (HLA-DR +). M1 macrophages secrete proinflammatory cytokines like tumor necrosis factor alpha (TNF-α) and various interleukins. This is thought to be the first-line response. As the injury progresses, usually after 48 h, there is a predominant Th2 response and macrophages get polarized to M2 phenotype (CD 163+). M2 macrophages can be a “double-edged sword.” On one side, the anti-inflammatory phenotype [mediated by transforming growth factor-β1 (TGF-β1) and interleukin-10 (IL-10)] is essential for adequate tissue repair; on the other side, it is a potential mediator of fibrosis and scarring (mediated by TGF-β1).10 If the injury persists or is severe enough, and the nephron has reached a point of no return, M2 macrophage shows profibrotic phenotype and the potential to transdifferentiate into myofibroblasts. These events in turn lead to a profibrotic milieu and the disease progresses to fibrosis.17 Current pharmacological treatments that present beneficial effects in retarding progression of CKD diminish macrophages infiltration in the kidney including the SGLT2 inhibitors.25
In response to injury, macrophages are recruited in the kidney from circulation. In the present study, we found that densities of CD68+, CD163+, and HLA-DR+ macrophages significantly increase in response to ATI as compared to MCD. Similar findings were noted by Palmer et al. evaluating interstitial macrophages in ATI. In addition, they also demonstrated M2 macrophages localized close to the tubular basement membrane of injured proximal tubule cell ultrastructurally.24
Kim et al. performed the first human study demonstrating the possible role of macrophages in the injury and repair phases of AKI.21 They also found that the infiltration of both CD 68+ and CD163+ macrophages increased significantly in ATI, with the density of the CD68+ macrophages being significantly higher in advanced-stage AKI. We also found strong and positive correlation between severity of tubular injury and densities of CD 68+ and CD163+ macrophages.
There was no significant correlation with density of HLA-DR + macrophages and the severity of ATI in our study, probably because the biopsies were done late in the course of the disease, where M1 macrophages are thought to be the first responders to injury.10
In the present study, we noted that the patients with complete recovery had a slightly higher densities of CD68+ and CD163+ macrophages when compared with the partial recovery group; however, this difference was not statistically significant. These findings reinforce the fact that inflammatory or wounding response to injury is essential for recovery in the first place. Injuries which do not show adequate inflammatory response do not heal. Later, the imbalance between M1 and M2 macrophages is believed to decide the fate of the healing process to recovery or fibrosis.17 Kim et al. found that persistence of CD163+ macrophages predicted nonrecovery.21 In the present study, we did not find any association between the densities of CD68+ and CD 163+ macrophages and the recovery profile. This can be explained by the longer duration of follow-up and the difference in defining the recovery in the study by Kim et al.21
Conclusion
Presently, there is no objective grading system of ATI. The system we propose is simple and can be used, as it correlates with severity of injury, namely serum creatinine. Though our study has not shown a correlation of these scores and the macrophage response to recovery patterns, which is driven by multiple factors, we believe that similar studies with larger numbers and the study of more patterns of injury could help evolve scoring systems for prediction of recovery. Age >60 years, hypertension, diabetes, and chronicity score on biopsy correlated with partial and delayed recovery.
Acknowledgement
Dr. Vikram Singh, Faststat for the statistical analysis.
Conflicts of interest
There are no conflicts of interest.
References
- A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: Part 1: Definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27:4263-72.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-84.
- [CrossRef] [PubMed] [Google Scholar]
- The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81:477-85.
- [CrossRef] [PubMed] [Google Scholar]
- Renal recovery after acute kidney injury. Intensive Care Med. 2017;43:855-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pathologic perspectives on acute tubular injury assessment in the kidney biopsy. Semin Nephrol. 2018;38:21-30.
- [CrossRef] [PubMed] [Google Scholar]
- Renal histopathologic findings associated with severity of clinical acute kidney injury. Am J Surg Pathol. 2018;42:625-35.
- [CrossRef] [PubMed] [Google Scholar]
- Macrophage phenotype in kidney injury and repair. Kidney Dis (Basel). 2015;1:138-46.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of immune cells in acute kidney injury and repair. Nephron. 2017;137:282-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of macrophages and related cytokines in kidney disease. Front Med (Lausanne). 2021;8:688060.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int. 2017;91:787-9.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of clinical characteristics and histologic descriptions of acute tubular injury. Kidney Int Rep. 2020;5:1993-2001.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Histological features of acute tubular necrosis in native kidneys and long-term renal function. Ren Fail. 2008;30:667-73.
- [CrossRef] [PubMed] [Google Scholar]
- Histological characteristics of acute tubular injury during delayed graft function predict renal function after renal transplantation. Physiol Rep. 2019;7:e14000.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Correlation of acute tubular injury in reperfusion biopsy with renal transplant outcomes. Clin Transplant. 2016;30:836-44.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical staining for proliferation antigen as a predictor of chronic graft dysfunction and renal graft loss. Nephron. 2002;92:738-42.
- [CrossRef] [PubMed] [Google Scholar]
- Archetypal analysis of injury in kidney transplant biopsies identifies two classes of early AKI. Front Med (Lausanne). 2022;9:817324.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology of renal recovery after acute renal failure. Curr Opin Crit Care. 2006;12:544-50.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term follow-up of patients after acute kidney injury: Patterns of renal functional recovery. PLoS One. 2012;7:e36388.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Recovery of kidney function after acute kidney injury in the elderly: A systematic review and meta-analysis. Am J Kidney Dis. 2008;52:262-71.
- [CrossRef] [PubMed] [Google Scholar]
- M2 macrophages predict worse long-term outcomes in human acute tubular necrosis. Sci Rep. 2020;10:2122.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317-26.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The role of M2 macrophages in the progression of chronic kidney disease following acute kidney injury. PLoS One. 2015;10:e0143961.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Quantification and localization of M2 macrophages in human kidneys with acute tubular injury. Int J Nephrol Renovasc Dis. 2014;7:415-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int J Mol Sci. 2020;21:3798.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







