Translate this page into:
HLA Desensitization Based on Results of the Luminex Technique in Kidney Transplant – A Single-center Experience
Address for correspondence: Dr. S.B. Bansal, Department of Nephrology and Kidney Transplantation, Medanta Kidney and Urology Institute, Medanta Medicity, Sector 38, Gurgaon - 122 001, Haryana, India. E-mail: drshyambansal@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
There is little experience of human leucocyte antigen (HLA) desensitization in India based on the Luminex single-antigen bead (SAB) testing. We retrospectively analyzed our patients, who underwent HLA desensitization based on Luminex SAB results.
Method:
Between 2014 and 2018, patients with complement-dependent cytotoxicity cross-match (CDC-XM) negativity but flow cytometry crossmatch (FC-XM) positivity were further analyzed with Luminex SAB for donor-specific antibodies (DSAs). A total of 12 patients who had DSA mean fluorescent intensity (MFI) of >1000 and <10,000 were included in the study. Our protocol for desensitization consisted of plasmapheresis (PP) followed by low dose intravenous immunoglobulin (IV IG) 100 mg/kg and induction with antithymocyte globulin (ATG). Patients were taken for transplant when either MFI was <1000 and/or FC-XM was negative.
Results:
All 12 patients were first transplant and 10 had a history of some sensitizing event; pregnancy in 4, blood transfusions in 4, and both in 2 patients. FC-XM was positive for T-cell in 4, B-cell in 6, and both in 2 patients. On evaluation by Luminex SAB, 6 patients had MFI from 1000 to 2000, and 6 had MFI of >2000. All underwent desensitization successfully. Two patients had an increase in posttransplant DSA titers requiring posttransplant PP. The mean follow-up was 26.6 ± 13.9 months. On follow-up, only one patient developed acute T cell-mediated rejection 1 year after transplant, which responded to pulse steroids. There was no graft or patient loss until the last follow-up.
Conclusion:
This study shows that HLA desensitization is feasible and successful in the Indian setting if patients are properly selected.
Keywords
HLA desensitization
kidney
living donor
Luminex
Introduction
A kidney transplant is considered the best treatment for most patients with kidney failure. There are many barriers to kidney transplantation and one of them is the presence of antibodies against human leukocyte antigens (HLA) in recipients particularly against corresponding donor labeled as donor-specific antibodies (DSAs). There are various tests to detect these antibodies, i.e., complement-dependent cytotoxicity cross-match (CDC-XM), flow cytometry crossmatch (FC-XM), and recently developed solid-phase assays like Luminex.[1234] The availability of solid-phase assay testing has improved the sensitivity and specificity for the detection of anti-HLA antibodies.[14] One such platform is the Luminex assay, in which purified HLA antigens are immobilized on solid beads and antibodies in recipients against HLA are reported as mean fluorescence intensity (MFI) which is a semi-quantitative measure of antibody levels.[4] With the advent of Luminex single-antigen bead (SAB) testing, it is possible to detect even weak DSAs. The removal of these DSAs, i.e., desensitization is associated with better outcomes as compared to those remaining on dialysis or Waiting list for transplant.[56]
Kidney transplant in India is mainly performed from living donors and there is limited availability of the related donors. There are few options for sensitized patients: One option is a paired kidney exchange (PKE) program, however, there is no regional or national PKE program in India and numbers are less in most individual centers limiting the feasibility of this option.[7] Kute et al. have been able to do a large number of PKE in a single-center due to the availability of many pairs,[8] so the only other option in the absence of alternative donor is desensitization. Montgomery et al. has shown better outcomes in patients with desensitization as compared to those remaining on dialysis on the waiting list for transplantation.[6] Desensitization can be attempted in many patients with low to moderate strength DSAs after careful evaluation of risks and benefits.
There are various protocols for HLA desensitization including high dose intravenous immunoglobulin (IVIG) alone, High dose IVIG with rituximab or plasmapheresis (PP) with low dose IVIG with or without rituximab, and induction with rabbit antithymocytic globulin (ATG).[6910] There is hardly any Indian data about desensitization based on Luminex SAB, except from our group of three patients and another recent study, but both had short follow up.[1112] We retrospectively analyzed the results of all desensitization performed at our center after the availability of the Luminex SAB technique after 2014.
Methods
This is a retrospective study of patients who underwent HLA desensitization at our center after the availability of Luminex SAB testing in 2014. Between April 2014 and December 2018, 12 out of 825 transplanted patients with DSA MFI >1000, who underwent HLA desensitization at our center were included in the study. Two patients in whom the desensitization was unsuccessful were not included. The approval of the ethics committee of the hospital was obtained (reference no: MICR-1104/2020).
CDC-XM was performed using isolated donor T-lymphocytes and B-lymphocytes from peripheral blood. FC-XM was performed using three-color flowcytometric (BD FACS Verse) and anti-human immunoglobin G (IgG) (Jackson ImmuoResearch Laboratories, USA) after discriminating T and B cells using CD3 and CD22 (BD Biosciences, USA). Single antigen bead (SAB) was performed using Life codes® LSA Class I and Class II kits (Immucor, Inc., GA, USA).[13] Patients who were CDC-XM negative but FC-XM positive were further analyzed with Luminex SAB to know the presence and strength of DSAs. Our mean channel shift (MCS) control for positive flow crossmatch for T-cell was 26 and for B-cell was 110. A sensitization history of blood transfusion, pregnancies, and the previous transplant were recorded in all participants.
We initially selected those patients for desensitization who had Luminex DSA MFI of >1000 and <2000. Later on, some patients with MFI from 2000–10000 were also considered for desensitization according to their overall immunological profile and other factors like age, affordability, etc., Patients were considered for desensitization after their written and informed consent explaining them of all complications including higher risks of rejection, increased cost, and possible graft loss.
Our protocol for desensitization consisted of PP 1.5 volume by double filtration on alternate days by centrifugation machine and low dose IV IG 100 mg/kg after each PP. Luminex SAB testing was repeated as required. The Patients were taken for the transplant, when DSA MFI was <1000 and/or FC-XM was negative. All patients received induction with thymoglobulin at a dose of 1.5 mg/kg on alternate days for 2–3 doses except one patient who received Grafalon 6 mg/kg (rabbit ATG). Patients who were also ABO-incompatible (ABOi), received pretransplant rituximab 200 mg single dose 2-3 weeks before transplant as part of our ABOi transplant protocol.
Immunosuppression was started a day before the transplant, which consisted of oral tacrolimus (TAC), 0.1 mg/kg/day in two divided doses, and oral mycophenolate (MMF)/MMF sodium 1000/720 mg twice daily. TAC levels were aimed at 8–12 ng/mL in the first 3 months, 6–8 ng/mL next 3 months, and 4–6 ng/mL thereafter. All patients received pulse methylprednisolone (MP) 500 mg perioperatively, followed by oral prednisolone which was gradually tapered to 5–7.5 mg by the end of 3 months. All patients received 3-months prophylaxis for CMV with valganciclovir and 6-months prophylaxis for pneumocystis with trimethoprim/sulfamethaxazole. Patients were followed up twice a week in the first month, once a week in the second month, and once in 2 weeks in the third month, and subsequently, once a month up to 1 year and once in 2 months thereafter. Due to financial constraints, we did not routinely perform DSAs in these patients posttransplant unless there was either a rise in serum creatinine or a decrease in urine output. All adverse events including new-onset diabetes after transplant (NODAT), infections, acute rejections (AR), graft loss, and death were noted.
In patients with graft dysfunction, after excluding TAC toxicity and obstruction, a kidney biopsy was performed unless contraindicated. All biopsies were processed for light microscopy (LM) and immunofluorescence (IF) including C4d. Our protocol of treatment for an acute TCMR is to give injection MP 500 mg once daily for 3–4 days which is followed by an injection thymoglobulin 1.5 mg/kg alternate days for 3–5 doses in event of poor response. The protocol for the treatment of acute AMR is PP and low dose IVIG 100 mg/kg after each PP for 3–7 sessions depending upon the response. All patients were followed for a minimum of 1 year.
Results
Out of 12 patients, five were male and 2/12 donors were male. All the patients were first transplant recipients and 10 patients had a history of some sensitizing events; 4 blood transfusions, 4 received had pregnancies, and 2 had history of both. The mean age of recipients was 39.4 ± 14.4 years, mean HLA mismatch was 3.3 ± 1.15, and mean duration of dialysis was 8.8 ± 15.2 months. The basic disease was unknown in 7 patients, and one patient each had diabetes mellitus, postpartum cortical necrosis, obstructive uropathy, antineutrophil cytoplasmic antibody-associated vasculitis (ANCA vasculitis), and reflux nephropathy.
CDC-XM was negative in all 12 patients, FC-XM was positive in all the patients with 4 had isolated B cell positivity, 6 had isolated B-cell positivity and 2 had both T and B-cell positivity [Table 1]. The mean MCS for T-cell was 98.8 ± 31.5 and the mean MCS for B-cell was 183.1 ± 32.5. On further evaluation by Luminex SAB testing, all these patients had DSAs: six patients had MFIs between 1000 to 2000, and the remaining six had MFI >2000. One patient had MFI as high as 7195 and six patients had multiple DSAs. [Table 1].
| Serial no. | FCXM (MCS) T-cell | FCXM (MCS) B-cell | DSA/MFI (pretransplant) | Treatment | Posttreatment MFI/FCXM |
|---|---|---|---|---|---|
| 1. | Positive (67) | Negative | B*08:01/1400 | 1 PP, | 784 |
| 2. | Negative | Positive- 160 | DRB3*01/2280 | 3 PP and IVIG, | 134, Repeat FCXM negative |
| 3. | Positive (74) | Negative | A 24/1949 | 2 PP and IV IG | 34 |
| 4. | Negative | Positive (170) | DQB1 03/516 DPA1 02/1839 Cumulative MFI: 2354 |
2 PP and IVIG | Repeat FCXM negative |
| 5 | Positive (126) | Positive (140) | A 02:03/2943 | 2 PP | Repeat FCXM negative |
| 6.ABOI | Negative | Positive (198) | A 24:02/2407 | Rituximab+3-PP+IVIG | 855 |
| 7. | Positive (118) | Negative | DPA1/7195B44/2090 Cumulative MFI: 9285 |
7PP+IVIG+ (Rituximab+2PP post Tx) MFI increased from 689-5200 | MFI 689(DPA1),718(B44) |
| 8. | Negative | Positive (244) | DQB103:02/1867DQA1/05:01/1776 Cumulative MFI: 3643 |
2PP+IVIG | 43 (DQB1)83(DQA1) |
| 9. | Negative | Positive (160) | DPA101:03/1701DPA102:01/1141DPB102:01/1134 Cumulative MFI: 3976 |
1PP+IVIG | 1028(DPA1*01)-839(DPA1*02)-676(DPB1)FC-XM -negative |
| 10ABOI | Positive (138) | Positive (195) | DQ A1 01/2755 | Rituximab+9PP | 733 |
| 11. | Positive 70) | Negative | A*24:02/2029, DPA1*01:03/3297, DPB1*04:02/3229 Cumulative MFI: 8555 |
3PP+IVIG | FCXM NegativeA*24:02 (1344)DPA1 (108) DPB1 (358) |
| 12. | Negative | Positive 9198) | DQB1 03:02- 1701 DQA1 05:01-697 Cumulative MFI: 2398 |
4 PP (+3pp post Tx in view increased posttransplant MFI1701>5706 and 697>2515) | DQB1 03:02- 540FXCM- negative |
CDC XM- complement-dependent cytotoxicity cross-match, FC-XM- flow cytometry cross-match, MFI-mean fluorescent antibody level, PP- plasmapheresis, IVIG-intravenous immunoglobulin, ATG- anti-thymocyte globulins, Tx- transplant
The endpoint of treatment was either DSA MFI <1000 and/or flow crossmatch negativity. Patients with MFI between 1000–2000 required 1–2 PP sessions and patients with MFI value of >2000 required 2–3 PP sessions to achieve the MFI values <1000 or negative FC-XM except patient number 12, who required four sessions of PP and patient number 7 with maximum MFI of 7195 who required 7 PP sessions. [Table 1]. Patient number 6 and 10 were also ABOi transplants with anti-blood group titers of 64 and 512, respectively but with relatively low strength DSA MFI <3000. The number of PP sessions for these two patients was predominantly guided by anti-blood group titers rather than MFI and patient number 10 required 9 sessions of PP. The DSA MFI titers were also achieved <1000 pretransplant [Table 1]. Posttransplant DSA were monitored in two patients (number 7 and 12) who were difficult to desensitize, and both had an increase in posttransplant DSA titers requiring posttransplant PP. [Table 1].
The mean follow-up of patients was 26.6 ± 13.9 months. Till the last follow-up, one patient had an episode of borderline TCMR after 1 year which improved with pulse MP. Three patients had biopsies for the asymptomatic rise in creatinine and all had patchy acute tubular necrosis (ATN) with no evidence of rejection. One patient developed transient CMV viremia (2500 copies/mL) on follow-up, which was done as a part of leukopenia work up and it resolved with a reduction in MMF dose to half. One patient developed lymph node tuberculosis (TB) 6 months after transplant and it improved with antitubercular treatment (ATT). Two patients developed urinary tract infection (UTI) posttransplant, which improved with antibiotics. All patients were doing well until the last follow-up.
The economics of the protocol
Most of our patients (11/12) used thymoglobulin as induction at a dose of 3-4.5 mg/kg, the cost of which is 20,000 for 25 mg vial, and the majority required 150-200 mg which means 150000 to 200000 INR. Only one patient received Grafalon at a dose of 6 mg/kg, the cost of which is 36000/100 mg vial, which is roughly 200000 INR. All patients also received intravenous immunoglobulin, 100 mg/kg after each PP, which costs INR 8,000 per 100 mg and most patients received 2-3 doses of IVIG. Besides, the cost of cascade PP at our center is 50,000/session, which includes the cost of the session and replacement fluids and the mean PP session were 3.2, although most patients required 2–3 sessions of PP and 2–3 doses of IVIG, except two patients, who required 7 and 9 sessions of PP due to difficult HLA desensitization and high ABOi titer, respectively. The average cost increase beyond normal expenditure including induction was approximately INR 4 lacs per individual.
Discussion
In this study, we successfully desensitized 12 HLA incompatible patients and only one patient had an episode of acute T cell-mediated rejection which responded to pulse steroids. All these patients were CDC-XM negative but FC-XM positive with DSA MFI ranging from 1000 to 10000. Initially, we did desensitization in patients who had single DSA with MFI <2000 but with the increasing experience we performed successful desensitization in patients with higher MFIs and multiple DSAs.
According to US data, approximately 15% of patients, waitlisted for transplant are sensitized to HLA by previous transplants, pregnancies, and/or blood transfusions.[5] Currently, there are two protocols of desensitization for HLA incompatible patients: One is high dose IVIG (2 g/kg) monthly, with or without rituximab used initially by Cedars Sinai group, predominantly in deceased donor transplantation.[9] The second protocol is PP + low dose IVIG (100 mg/kg) used by the Mayo Clinic and John Hopkins.[610] Stegall et al. in one of the initial studies compared two different doses of IV IG, one group received only high dose IVIG (2 g/kg) and another group received low dose IVIG (100 mg/kg) along with PP and rituximab in patients with CDC-XM positivity. He found that a combination of low dose IVIG with PP was more successful in making crossmatch negative compared to high dose IVIG alone (84% vs. 38%, P < 0.01).[10] We followed the protocol of PP plus low dose IVIG and induction with ATG. We did not use pretransplant rituximab in desensitization except in two patients with ABO-incompatible kidney transplant protocol and one patient with posttransplant rebound in MFI from 689 pretransplant to 5214 on POD 6.
It is reported that patient survival is better in patients with HLA desensitization as compared to those on the waiting list or dialysis. Segev et al. in their multicentric study has shown that 8-year patient survival was 76.5%, 62.9%, and 43.9%, respectively in HLA incompatible group, patients on the waitlist for transplant group and waitlist only group (P < 0.001). The survival was best in the positive Luminex negative flow (PLNF) group (89.2%), intermediate in positive flow, negative CDC (PFNC) group (76.1%), and least in positive CDC CM group (71%).[14] We excluded patients with CDC-XM positivity.
Graft survival is also better in these patients. Bentall et al.[15] in 102 cross-match positive patients have shown that 5-year graft survival of 70% as compared to 90% in crossmatch negative patients, however, 40% of patients in the treatment group were CDC positive, who had a very high risk of AMR and subsequent graft loss. They have also shown that patients with DSA MFI >3000 have a higher risk of rejection as compared to patients with MFI <3000. We mostly included patients with MFI of <3000 and excluded those with strong DSAs of >10,000 or multiple DSAs of >5000 and achieved good outcomes. Our experience shows that single plasmapheresis can reduce the MFI approximately by 1000–1500.
Lefaucheur and colleagues[16] designed an algorithm based on the sensitization of their patients for transplant and desensitized only those patients for whom donors could not be found despite enrolling them in PKE national waiting list and they could minimize transplanting highly sensitized patients.
We suggest that HLA incompatible transplants should only be attempted in patients without the option of PKE or alternative donor, but at the same time, patients with low/moderate strength DSAs with or without FC-XM positivity should not be denied the benefits of transplantation due to lack of these options. Segev et al. in a US national survey of 125 centers has shown that only 71%, 50%, and 18% centers perform desensitization in patients with PLNF, PFNC, and positive CDC-XM, respectively.[17] This study is relevant in the Indian scenario and we suggest that centers should decide their threshold of desensitization based on infrastructure support, experience, and risk appetite. We have designed an algorithm based on our experience about desensitization approach in the Indian scenario [Figure 1].
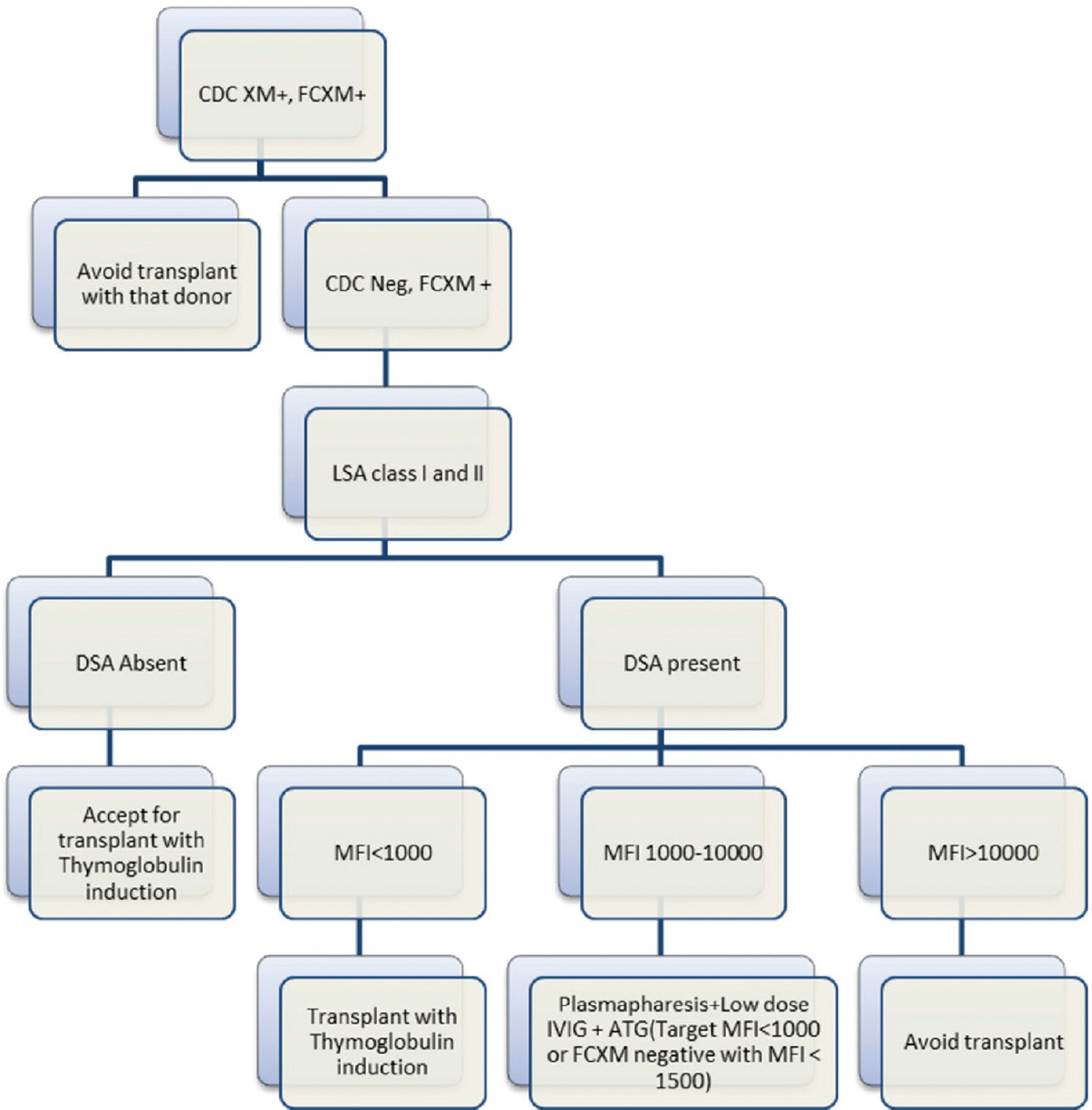
- Proposed desensitization flow chart
Our study has certain limitations. Firstly, the number of patients is less and we included only those patients who were at relatively low risk of developing AMR i.e., CDC-XM negative with MFI <10000 and preferably <3000. However, this was the initial experience and with increasing experience, we are now including patients with higher MFIs. Secondly, we did not routinely monitor posttransplant DSA except in two patients with difficult desensitization as this is a costly test and difficult to do repeatedly without a clinical indication. The strength of our study is that this is one of the initial experiences of desensitization using Luminex single bead assay in an Indian setting with good follow up. Another Indian study by Kute et al.[18] performed desensitization using bortezomib + PP + IVIG + ATG, but immunological evaluation in that study was inadequate and did not include Luminex SAB testing.
To summarize, this study shows that HLA desensitization is possible and successful in Indian circumstances if we choose our patients carefully and it can provide a new lease of life to these patients who would otherwise not get a kidney due to lack of large kidney paired exchange or deceased donor program.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Dr. Vijay Kher, Dr. Prasun Ghosh.
References
- Utility of HLA antibody testing in kidney transplantation. J Am Soc Nephrol. 2015;26:1489-502.
- [Google Scholar]
- Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735-9.
- [Google Scholar]
- Flow cytometric crossmatching in primary renal transplant recipients with a negative anti-human globulin enhanced cytotoxicity crossmatch. J Am Soc Nephrol. 2001;12:2807-14.
- [Google Scholar]
- Clinical relevance of anti-HLA donor-specific antibodies detected by Luminex assay in the development of rejection after renal transplantation. Transplantation. 2012;94:338-44.
- [Google Scholar]
- Approach to the highly sensitized kidney transplant candidate. Clin J Am Soc Nephrol. 2016;11:684-93.
- [Google Scholar]
- Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365:318-26.
- [Google Scholar]
- Paired kidney exchange transplantation: Maximizing the donor pool. Indian J Nephrol. 2015;25:349-54.
- [Google Scholar]
- Impact of single-centre kidney paired donation transplantation to increase the donor pool in India. Transpl Int. 2017;30:679-88.
- [Google Scholar]
- Use of intravenous immune globulin and rituximab for desensitization of highly HLA-sensitized patients awaiting kidney transplantation. Transplantation. 2010;89:1095-102.
- [Google Scholar]
- A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant. 2006;6:346-51.
- [Google Scholar]
- Successful renal transplantation across HLA barrier: Report from India. Indian J Nephrol. 2017;27:210-4.
- [Google Scholar]
- Renal transplantation in HLA sensitized patients: Traversing the immunological barrier. Ther Apher Dial. 2019;20:1744.
- [Google Scholar]
- CLSI. Detection of HLASpecific Alloantibody by Flowcytometry and Solid Phase Assay; Approved Guideline. In: CLSI document I/LA29A. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
- [Google Scholar]
- Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med. 2016;374:940-50.
- [Google Scholar]
- Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant. 2013;13:76-85.
- [Google Scholar]
- Mastering the risk of HLA antibodies in kidney transplantation: An algorithm based on pretransplant single-antigen flow bead techniques. Am J Transplant. 2011;11:1592-8.
- [Google Scholar]
- Incompatible live-donor kidney transplantation in the United States: Results of a national survey. Clin J Am Soc Nephrol. 2011;6:2041-6.
- [Google Scholar]
- Desensitization protocol for highly sensitized renal transplant patients: A single-center experience. Saudi J Kidney Dis Transpl. 2011;22:662-9.
- [Google Scholar]







