Translate this page into:
Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors for Anemia in Dialysis-Dependent Chronic Kidney Disease: Systematic Review and Meta-Analysis of Randomized Controlled Trials
Corresponding author: Soumyadeep Bhaumik, Meta-Research and Evidence Synthesis Unit, The George Institute for Global Health, Delhi, India. E-mail: sbhaumik@georgeinstitute.org
-
Received: ,
Accepted: ,
How to cite this article: Tyagi J, Kaur M, Ingale S, Ramachandran R, Meena P, Bajpai D, et al. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors for Anemia in Dialysis-Dependent Chronic Kidney Disease: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Indian J Nephrol. 2025;35:198-216. doi: 10.25259/ijn_379_23
Abstract
Hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs) are oral drugs for patients with renal anemia. This study aimed to synthesize evidence on HIF-PHIs for anemia in dialysis-dependent chronic kidney disease (DD-CKD) patients. We searched PubMed, CINAHL, and Cochrane Central Register of Controlled Trials databases and trial registries for randomized controlled trials (RCTs) reporting HIF-PHIs versus erythropoietin-stimulating agents (ESA) for anemia in DD-CKD patients. Two authors independently conducted screening, data extraction, and assessed risk of bias. We used RevMan 5.3 software for meta-analysis using standard methods. Certainty of evidence was assessed by Grading of Recommendations, Assessment, Development, and Evaluations (GRADE). We included 20 RCTs involving 14,999 patients with anemia of kidney disease. The studies included roxadustat (n = 9), daprodustat (n = 5), vadadustat (n = 2), molidustat (n = 2), enarodustat (n = 1), and desidustat (n = 1). Overall, daprodustat as an alternative to ESAs reported a substantial net benefit while roxadustat showed more damage than benefit as compared to ESAs. While other HIF inhibitors demonstrated little to no difference or small benefit, daprodustat reduces the need for intravenous iron supplementation up to 52 weeks as compared to ESAs [Odds Ratio (OR): 0.77 (95% CI 0.53–1.13); p = 0.18; two studies; 674 participants; moderate certainty evidence]. Roxadustat increased treatment-emergent adverse events up to 6–52 weeks as compared to ESAs [OR: 1.45 (95% CI 1.08–1.96); p = 0.01; six studies; 1715 participants; moderate certainty evidence]. The study provided evidence on the use of HIF-PHIs for treating renal anemia in DD-CKD patients as an alternative to ESAs.
Keywords
Chronic kidney disease
Renal anemia
Non-dialysis dependent
Hypoxia-inducible factor prolyl hydroxylase inhibitors
Introduction
Chronic kidney disease (CKD) is a state of progressive loss of kidney function. It affects around 697.5 million people globally and is the 12th leading cause of death.1 Anemia is a frequent occurrence in all stages of CKD, and its prevalence increases with the advancement of the CKD stage.2 Its presence is associated with poor clinical outcomes, including increased fatigue, increased risk of hospitalization, progression to dialysis dependence, reduced quality of life (QoL), increased morbidity, and all-cause mortality.3 Besides erythropoietin (EPO) deficiency, chronic inflammation and impaired iron homeostasis also play a role in the pathophysiology of anemia in CKD and are important targets for management.4 Proper management of anemia in CKD improves patient outcomes. Currently, the most frequently used management options are erythropoiesis-stimulating agents (ESAs) along with adjunct iron therapy and rescue blood transfusions. ESAs however may lead to adverse effects such as cardiovascular events, thrombosis, hypertension, and all-cause mortality. Hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs) are a new therapy option for anemia in CKD. The drugs mimic hypoxia and stimulate EPO production to correct the anemia.4 The HIF-PHIs, like the ESAs, eventually increase EPO levels. In theory, HIF-PHIs offer the advantages of oral bioavailability, close to physiologic EPO stimulation, and better utilization of available iron. There is insufficient literature on the long-term effects of HIF-PHIs.5,6
Limited evidence exists regarding initiation, monitoring, substitution/adjunct therapy, and withdrawal of HIF-PHIs.7 The interpretation of available literature also varies, as evident in the conflicting regulating approvals for HIF-PHIs.8 The evidence on HIF-PHIs is rapidly evolving and needs to be synthesized for assessing the benefits versus harms. Drug-makers are looking to expand the HIF-PHI market in India, and in March 2022, desidustat was approved for the treatment of anemia in adults with CKD who are either on dialysis or not on dialysis.9 As such, there is a need for clinical practice guidelines on HIF-PHIs. We aimed to evaluate the efficacy and safety of HIF-PHI in dialysis-dependent chronic kidney disease (DD-CKD) patients with anemia to support the development of clinical practice guidelines on this topic in South Asia.10
Our systematic review synthesizes evidence individually for each molecule of HIF-PHIs for DD-CKD patients, which is different from other systematic reviews on HIF-PHIs that have pooled data from all HIF-PHI molecules together or have been conducted for a single HIF-PHI molecule, but data from DD-CKD and non-dialysis-dependent CKD (NDD-CKD) has been pooled together. Our analysis is more nuanced and in alignment with how clinical practice is affected. It is well known that different HIF-PHI molecules have different safety profiles (the reason they are being developed) making them not interchangeable. Pooling data from all HIF-PHIs together might give a false sense of safety. The profile of DD-CKD and NDD-CKD patients are substantially different with their management being affected differently. A systematic review of HIF-PHIs in NDD-CKD patients is presented separately.
Methods
The review is reported in accordance with Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) 2020 guideline; the PRISMA checklist is presented in Appendix 1. The protocol was registered a priori in the Open Science Framework (OSF) (https://osf.io/a6b8r).
We included studies which met the following criteria:
-
Population/participants: Adult patients (≥18 years) of CKD with a diagnosis of anemia and on any form of dialysis (hemodialysis or peritoneal dialysis). We excluded studies on patients with primary anemia due to systemic causes like bone marrow aplasia or pure red cell aplasia, thalassemia major, sickle cell disease or myelodysplastic syndrome, and untreated pernicious anemia or anemia secondary to other causes such as blood loss due to gastrointestinal (GI) bleeding, cancer, and infectious diseases. If a study involved both adults and children or adolescents, then we included only if disaggregated data on adults was reported in the full text. Anemia and CKD diagnostic criteria used was as defined by the primary authors.
-
Intervention: HIF-PHI administered, including but not limited to: Daprodustat, Desidustat, Enarodustat, Molidustat, Roxadustat, and Vadadustat. We included studies irrespective of their dosage and frequency of administration.
-
Comparison: We included studies with any ESAs including but not limited to epoetin alfa or darbepoetin alfa administered by any route as a comparator.
-
Study designs: Randomized controlled trials.
-
Types of outcome measures: We included studies reporting the following outcomes:
Change in hemoglobin levels from baseline
All-cause mortality
Need for iron supplementation
Need for ESA
Health-related QoL (measured by any validated tool)
Fatigue (measured by any validated tool)
Incidences of major adverse cardiovascular events (MACE) and MACE plus (as defined by the author treatment emergent adverse events AEs, and patient requiring blood transfusion.
We did not include studies published in non-English languages (where a publicly available translation was not available) and which were available in abstract form only (with no full-length publication available). Authors of studies were not contacted for full texts. We did not restrict by publication date.
For the validated tools, all scales operate in the same direction and higher scores indicate greater satisfaction. We captured all time points (above six months), at which the outcomes were measured and determined by the included studies that were explicitly mentioned in the review report. Outcome time points were captured at baseline and up to the maximal time point available. However, there were few outcomes recorded at multiple time points; thus we assumed the maximal time point available to be equal to the length of follow-up if not specifically mentioned. We assessed outcome measures as per the following: up to 12 months as short term and greater than 12 months as long term.
An inclusive outcome measurement/definition approach was followed to enable capturing of maximal evidence, such that outcomes measured in terms of frequency/proportion or any other modality were included.
Information sources
Electronic database search
A search strategy was developed in PubMed, which was adapted for other electronic databases. The electronic databases searched were PubMed, EMBASE, CINAHL, The Cochrane Central Register of Controlled Trials, Trial registries (clinicaltrials.gov, World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), Clinical Trials Registry - India (CTRI), and Sri Lanka Clinical Trials Registry (SLCTR). All search strategies are presented in Appendix 2.
Other methods for searching
The guideline development group 1(GDG) members were contacted for identifying additional studies that potentially meet eligibility criteria. The reference lists of studies that meet eligibility criteria and those retrieved by other modalities of the search were manually screened for identifying newer studies.
Data collection and analysis
Selection of studies
At least two review authors independently screened the title and/or abstracts from the electronic database search for relevance using the web application Rayyan.11 This was followed by the full-text articles evaluation against the inclusion criteria by at least two review authors. Any discrepancies were resolved by consensus with the other review author.
Data extraction and management
At least two reviewers independently extracted data as per a predesigned data extraction form. Disagreements were resolved by consensus between two authors, with a third author acting as arbiter. Authors of studies were not contacted for additional data and only data as reported in published versions was included.
Assessment of risk of bias in included studies
Risk of bias was assessed by two reviewers independently. We used Cochrane Risk of Bias 1.0 tool developed by Cochrane.12
Measures of treatment effect
The measures of effect used depended on the type of outcome data.
For dichotomous outcomes (all-cause mortality, need for iron supplementation, need for ESA, incidence of MACE and/or MACE Plus, treatment emergent AEs, and patient requiring blood transfusion), OR with 95% confidence intervals (CI) were used.
For continuous outcomes (change in the hemoglobin level, health-related QOL, and fatigue), mean difference with 95% CI (where included studies report outcomes measured on the same scale) or standardized mean difference with 95% CI (where included studies report the same outcome measured differently) was used.
Unit of analysis issues
The unit of analysis was the individual participant.
Data synthesis
We summarized results of the included studies narratively and conducted meta-analysis where applicable, as per guidance in the Cochrane Handbook for Systematic Reviews of Interventions. Considering expected heterogeneity, we used a random-effects approach for meta-analysis. Conducting meta-analysis with a fixed-effect model in the presence of even minor heterogeneity may provide overly narrow CIs. We used the Chi2 test and the I2 measure to quantify heterogeneity, but we did not use these to guide the choice of model for meta-analysis.
Assessment of heterogeneity
Clinical and methodological heterogeneity was evaluated by generating descriptive statistics for trial, study population, intervention, outcome, setting, and characteristics such as the length of follow-up and more across all eligible trials that compare each pair of interventions. This assessment of clinical and methodological heterogeneity was supplemented by information regarding statistical heterogeneity. Heterogeneity of included studies of a particular intervention-outcome pair was assessed by standard methods.13
Assessment of reporting biases
Funnel plots were used to assess for the potential existence of study bias if enough studies (at least ten) were available using standard methods.13 Nonreporting of outcome bias was only done for studies that had a priori registrations or protocols available. Selective reporting within studies was also analyzed. We described and provided frequencies of outcomes not reported.
Dealing with missing data
Investigators for included studies were not contacted to obtain any missing numerical outcome data owing to the time frame in which the systematic review was being conducted. As such, when missing data is encountered, estimations were made as per methods described in the Cochrane Handbook (Chapter 10.12.2).
Certainty of evidence from trials
We used the GRADE approach to estimate certainty of evidence as per the GRADE handbook.14 We used the GRADE Pro GDT software (https://gradepro.org) to create a Summary of Findings (SoF) table for all primary outcomes. The SoF table presented a maximum of seven outcomes, including AEs in the SoF table. In the GRADE approach, certainty of evidence was classified as very low, low, moderate, and high by the consensus of the review team (involving at least two authors). Randomized controlled trials (RCTs) start with high quality rating. We reduced or downgraded the certainty of evidence based on the factors listed below, using the methods described in the GRADE handbook:
Five factors that can lower confidence in the estimate of an effect (i.e., lower the quality of evidence): study limitations (risk of bias), inconsistency of results, indirectness of evidence, imprecision and publication bias
Difference between protocol and full review
Patient requiring blood transfusion was not a priori outcome noted in the protocol. This was added to capture additional evidence reported in trials that could be useful for decision-making.
Results
We identified 838 studies from database searches, and following removal of 118 duplicates, we screened 720 records based on titles and/or abstracts. We retrieved full texts of 160 studies which were deemed to be potentially eligible for further examination. On full-text screening, 20 studies were included in this report. Figure 1 shows the PRISMA study selection flow chart. The list of excluded studies with reasons for exclusion at the full text level is presented in Appendix 3. We found 20 studies involving 14,999 anemia patients, assessing efficacy and safety of six HIF-PHI compounds in DD-CKD patients with anemia.15–34 The studies included roxadustat (n = 9), daprodustat (n = 5), vadadustat (n = 2), molidustat (n = 2), enarodustat (n = 1), and desidustat (n = 1). We found 14 trials conducted on ESA-conditioned patients, four on both ESA-conditioned and naïve patients, and two on ESA naïve. We found 11 studies including patients undergoing hemodialysis, one study on peritoneal dialysis, and eight studies on both peritoneal and hemodialysis. The treatment duration ranged from six weeks to four years. All characteristics of the studies are summarized in Appendix 4.
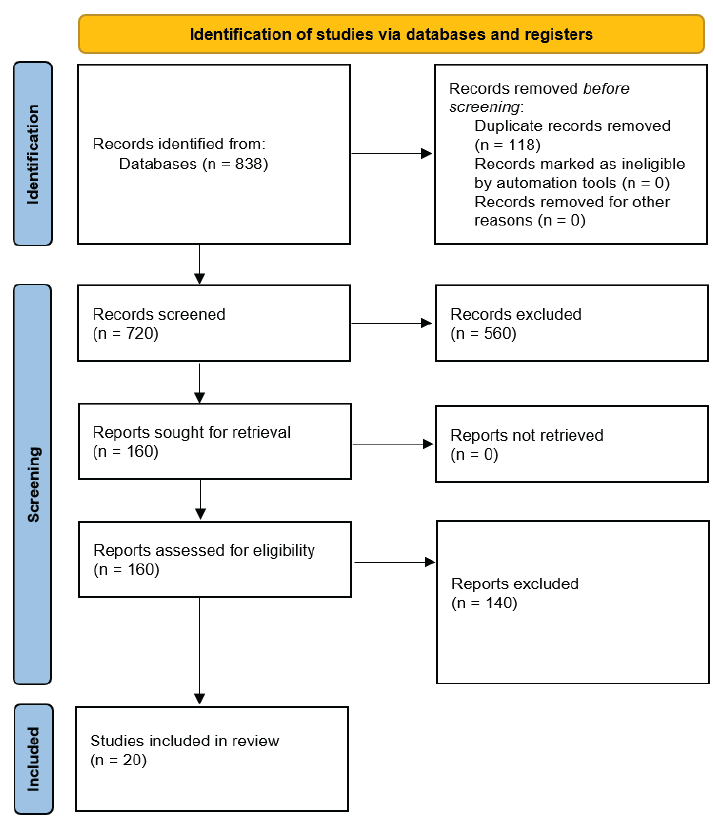
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart showing selection of studies.
Quality assessment of included studies
The risk of bias summary for the 20 included RCTs is presented in Figure 2.
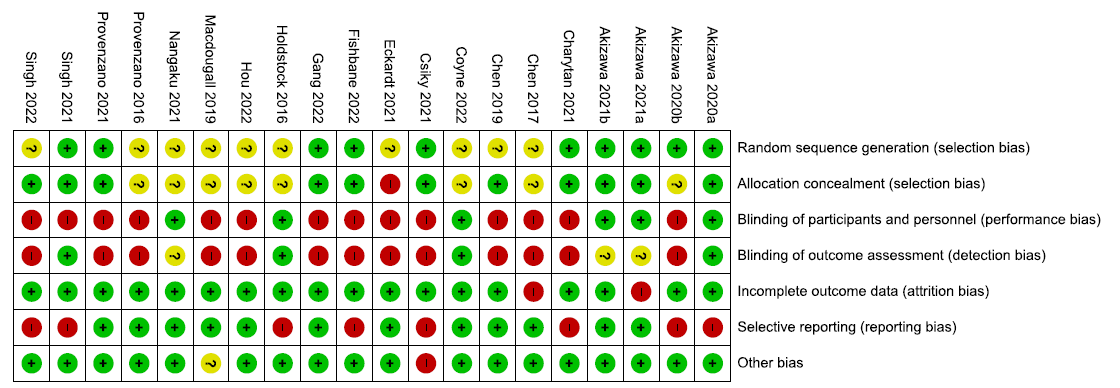
- Risk of bias summary for the included randomized controlled trials. Low risk of bias is signified by the green circles with ‘+’ symbols, Unclear risk of biase is signified by the yellow circles with ‘?’ symbol, and High risk of bias is signified by the red circles with ‘-’ symbol.
We used Cochrane Risk of Bias 1.0 tool to assess the risk of bias of all the included studies. Majority of the studies were judged at low or unclear bias for random sequence generation, allocation concealment, incomplete outcome data, and other biases. Fourteen studies were judged at high risk for blinding of participants and personnel, while 13 were considered to be at high risk of blinding of outcome assessors as a result of open-label design of the studies. Eight studies were judged at high risk for selective reporting as the studies did not report prespecified outcomes.
Meta-Analysis
All GRADE evaluations are presented in Appendix 5.
Effect of HIF-PHI on the change in hemoglobin levels from baseline
We found 19 studies reporting the effect of HIF-PHIs on the change in hemoglobin from baseline as compared to ESAs.
Effect of desidustat versus epoetin alfa on the change in hemoglobin levels from baseline up to 16–24 weeks
One study reported change in hemoglobin levels from baseline up to 16–24 weeks in desidustat as compared to epoetin alpha. Desidustat reported little or no difference in the hemoglobin levels from baseline up to 16–24 weeks as compared to epoetin alfa [mean difference (MD): 0.07 g/dL (95% CI -0.23-0.37); p = 0.65; 373 participants; very low certainty evidence].26 The forest plot is shown in Figure 3.
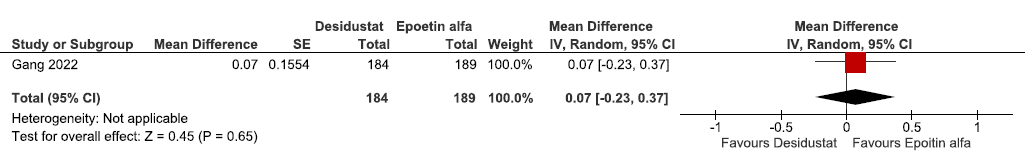
- Forest plot for desidustat versus epoetin alfa on the change in hemoglobin levels from baseline up to 16–24 weeks. CI: confidence intervals, SE: standard error, IV: inverse variance.
Effect of daprodustat versus ESAs (rhEPO/darbepoetin alpha/epoetin alpha) on the change in hemoglobin levels from baseline up to 52 weeks
Four studies reported the change in hemoglobin levels from baseline up to 52 weeks in daprodustat as compared to ESAs. The pooled results reported daprodustat had little or no difference in change in the hemoglobin levels from baseline up to 52 weeks as compared to ESAs [MD: 0.02 g/dL (95% CI -0.14, 0.18); p = 0.80; four studies; 3950 participants; low certainty evidence].18,22,33,34 The forest plot is shown in Figure 4.
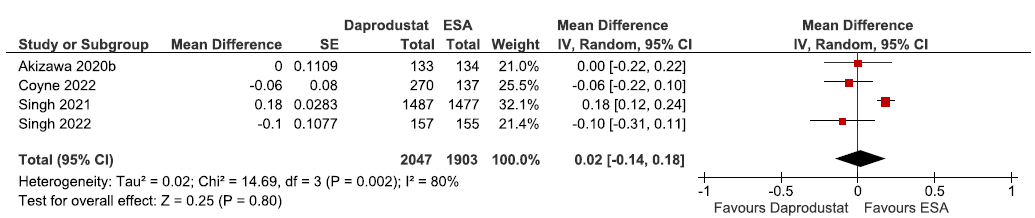
- Forest plot for daprodustat versus ESAs on the change in hemoglobin levels from baseline up to 52 weeks. CI: Confidence intervals, SE: Standard error, IV: Inverse variance, ESA: Eythropoiesis-stimulating agents.
Effect of enarodustat versus darbepoetin alpha on the change in hemoglobin levels from baseline up to 24 weeks
One study reported change in hemoglobin levels from baseline up to 24 weeks in enarodustat as compared to darbepoetin alpha. Enarodustat reduced the hemoglobin levels from baseline up to 24 weeks as compared to darbepoetin alpha (MD: -0.12 g/dL [95% CI -0.33–0.09]; p = 0.26; 172 participants; low certainty evidence).16 The forest plot is shown in Figure 5.
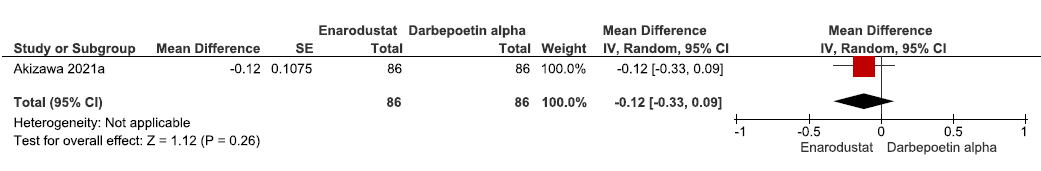
- Forest plot for enarodustat versus darbepoetin alpha on the change in hemoglobin levels from baseline up to 24 weeks. CI: Confidence intervals, SE: Standard error, IV: Inverse variance.
Effect of molidustat versus ESA on the change in hemoglobin levels from baseline up to 36 weeks
Two studies reported change in hemoglobin levels from baseline up to 36 weeks in molidustat as compared to ESAs. The pooled results reported molidustat lowered the hemoglobin levels from baseline up to 36 weeks as compared to ESAs. [MD: -0.17 g/dL (95% CI -0.43–0.10); p = 0.22; two studies; 379 participants; low certainty evidence].16,29 The forest plot is shown in Figure 6.
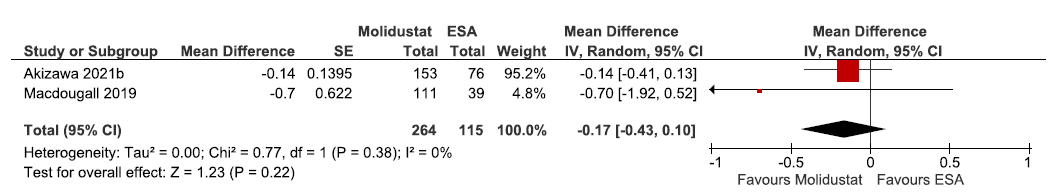
- Forest plot for molidustat versus ESA on the change in hemoglobin levels from baseline up to 36 weeks. CI: Confidence intervals, SE: Standard error, IV: Inverse variance, ESA: Eythropoiesis-stimulating agents, df: degrees of freedom.
Effect of roxadustat versus ESA on the change in hemoglobin levels from baseline up to 6–52 weeks
Nine studies reported change in hemoglobin levels from baseline up to 6–52 weeks in roxadustat as compared to ESAs. The pooled results reported roxadustat significantly raised the hemoglobin levels from baseline up to 6–52 weeks as compared to ESAs. [MD: 0.21 g/dL (95% CI 0.11–0.32); p < 0.0001; nine studies; 5553 participants; low certainty evidence].15,19–21,23,25, 28,31,32 The forest plot is shown in Figure 7.
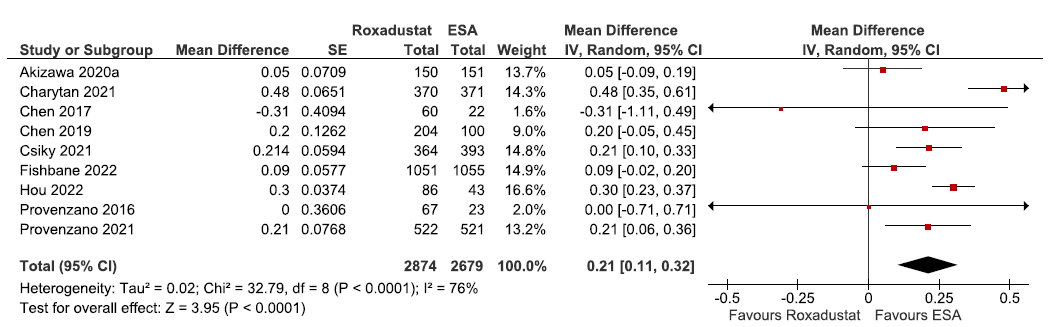
- Forest plot for roxadustat versus ESA on the change in hemoglobin levels from baseline up to 6–52 weeks. CI: Confidence intervals, SE: Standard error, IV: Inverse variance, ESA: Eythropoiesis-stimulating agents, df: degrees of freedom.
Effect of vadadustat versus darbepoetin alpha on the change in hemoglobin levels from baseline up to 52 weeks
Two studies reported change in hemoglobin levels from baseline up to 52 weeks in vadadustat as compared to darbepoetin alpha. The pooled results reported vadadustat lowered the hemoglobin levels from baseline up to 52 weeks as compared to darbepoetin alpha. (MD: -0.15 g/dL [95% CI -0.24 -0.07); p = 0.0006; two studies; 5553 participants; low certainty evidence).24,30 The forest plot is shown in Figure 8.
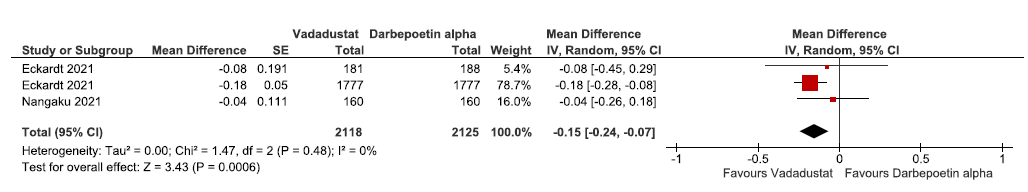
- Forest plot for vadadustat versus darbepoetin alpha on the change in hemoglobin levels from baseline up to 52 weeks. CI: Confidence intervals, SE: Standard error, IV: Inverse variance, df: degrees of freedom.
Effect of HIF-PHI on all-cause mortality
We found 20 studies reporting the effect of HIF-PHIs on all-cause mortality as compared to ESAs.
Effect of desidustat versus epoetin alfa on all-cause mortality up to 26 weeks
One study reported all-cause mortality up to 26 weeks in desidustat as compared to epoetin alpha. Desidustat reduced all-cause mortality up to 26 weeks as compared to epoetin alfa. [OR: 0.56 (95% CI 0.16–1.95); p = 0.36; 392 participants; very low certainty evidence].26 The forest plot is shown in Figure 9.
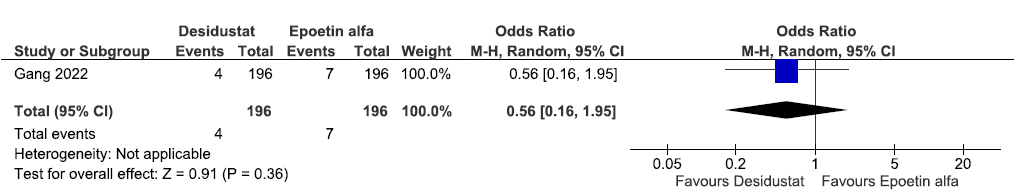
- Forest plot for desidustat versus epoetin alfa on all-cause mortality up to 26 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method.
Effect of Daprodustat Vs ESAs (rhEPO/Darbepoetin Alpha/Epoetin Alpha) on all-cause mortality up to 52 weeks
Five studies reported all-cause mortality up to 52 weeks in daprodustat as compared to ESAs. The pooled results reported daprodustat have little or no difference on all-cause mortality up to 52 weeks as compared to ESAs [OR: 0.98 (95% CI 0.82–1.16); p = 0.81; five studies; 4035 participants; low certainty evidence].18,22,27,33,34 The forest plot is shown in Figure 10.
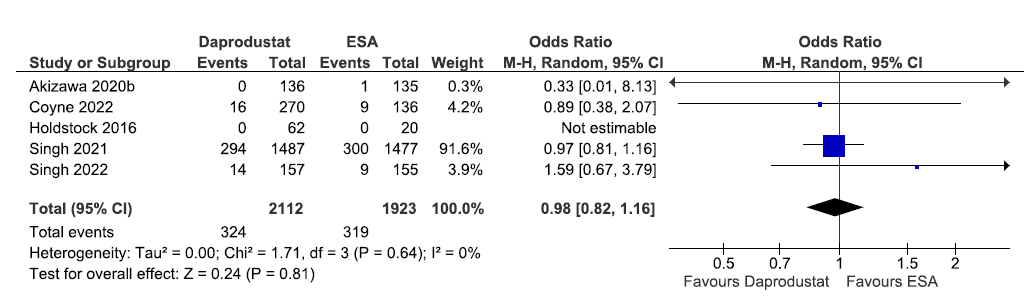
- Forest plot for daprodustat versus ESAs on all-cause mortality up to 52 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method, df: degrees of freedom.
Effect of enarodustat versus darbepoetin alpha on all-cause mortality up to 26 weeks
One study reported all-cause mortality up to 26 weeks in enarodustat as compared to darbepoetin alpha. There were none who experienced the all-cause mortality up to 26 weeks, to determine whether enarodustat made a difference as compared to darbepoetin alpha (OR: not estimable; 173 participants; very low certainty evidence).16 The forest plot is shown in Figure 11.
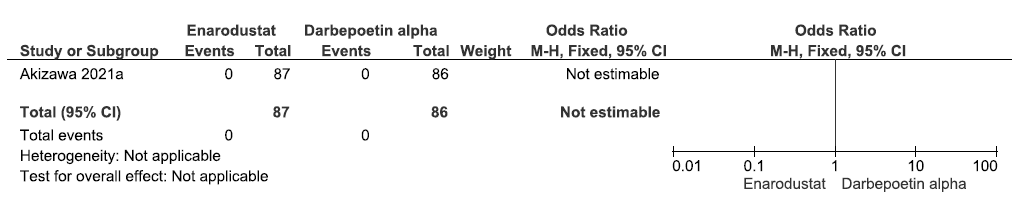
- Forest plot for enarodustat versus darbepoetin alpha on all-cause mortality up to 26 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method.
Effect of molidustat versus ESA on all-cause mortality up to 52 weeks
Two studies reported all-cause mortality up to 52 weeks in molidustat as compared to ESAs. The pooled results reported molidustat decreased the all-cause mortality up to 52 weeks as compared to ESAs [OR: 0.56 (95% CI 0.1–3.04); p = 0.50; two studies; 428 participants; very low certainty evidence].17,29 The forest plot is shown in Figure 12.
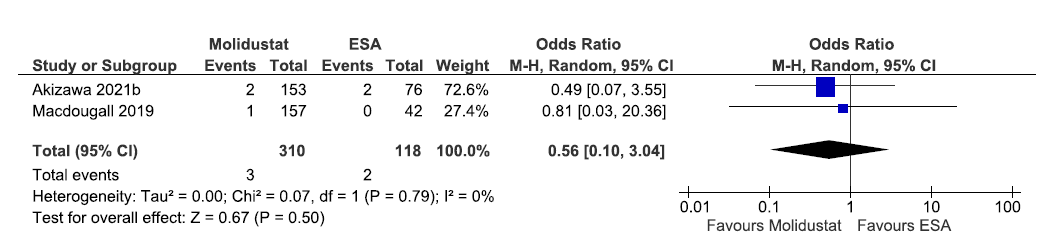
- Forest plot for molidustat versus ESA on all-cause mortality up to 52 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method, df: degrees of freedom.
Effect of roxadustat versus ESA on all-cause mortality up to 6–52 weeks
Six studies reported all-cause mortality up to 6–52 weeks in roxadustat as compared to ESAs. The pooled results reported roxadustat increased all-cause mortality up to 6–52 weeks as compared to ESAs [OR: 1.11 (95% CI 0.76–1.62); p = 0.60; six studies; 1715 participants; low certainty evidence].15,19–21,28,31 The forest plot is shown in Figure 13.
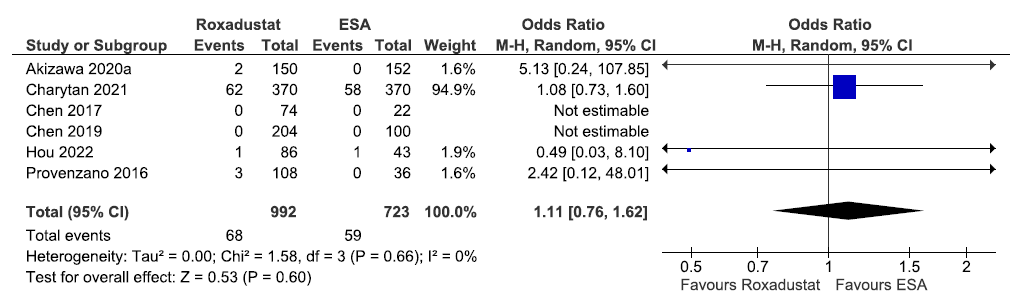
- Forest plot for roxadustat versus ESA on all-cause mortality up to 6–52 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method, df: degrees of freedom.
Effect of roxadustat versus ESA on all-cause mortality up to 108–209 weeks
Three studies reported all-cause mortality up to 108–209 weeks in roxadustat as compared to ESAs. The pooled results reported roxadustat increased all-cause mortality up to 108–209 weeks as compared to ESAs [OR: 1.13 (95% CI 0.96–1.33); p = 0.15; three studies; 3974 participants; very low certainty evidence].23,25,32 The forest plot is shown in Figure 14.
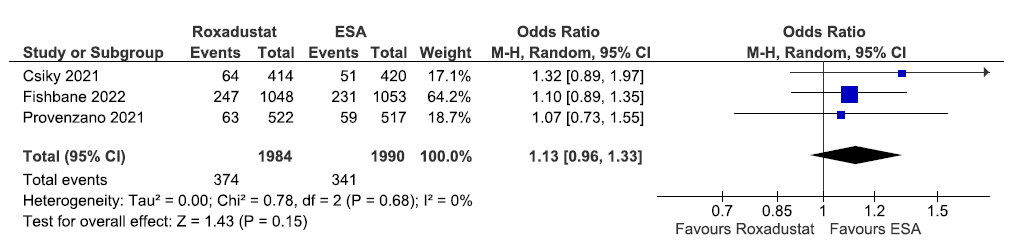
- Forest plot for roxadustat versus ESA on all-cause mortality up to 108–209 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method, df: degrees of freedom.
Effect of vadadustat versus darbepoetin alpha on all-cause mortality up to 52 weeks
One study reported all-cause mortality up to 52 weeks in vadadustat as compared to darbepoetin alpha. The pooled results reported vadadustat increased all-cause mortality up to 52 weeks as compared to ESAs [OR: 2.0 (95% CI 0.18–22.28); p = 0.57; 323 participants; very low certainty evidence].30 The forest plot is shown in Figure 15.
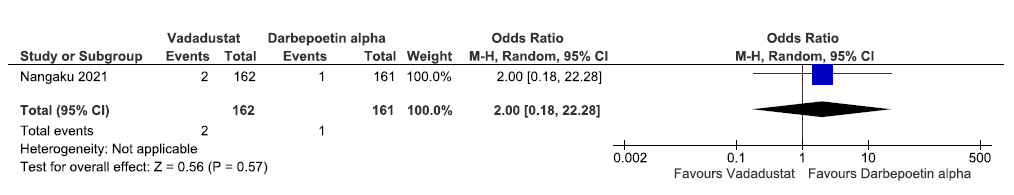
- Forest plot for vadadustat versus darbepoetin alpha on all-cause mortality up to 52 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method.
Effect of vadadustat versus darbepoetin alpha on all-cause mortality up to 116 weeks
One study reported all-cause mortality up to 116 weeks in vadadustat as compared to darbepoetin alpha. The pooled results reported vadadustat has little or no difference on all-cause mortality up to 116 weeks as compared to ESAs [OR: 1.0 (95% CI 0.83–1.21); p = 0.96; 3902 participants; very low certainty evidence].24 The forest plot is shown in Figure 16.
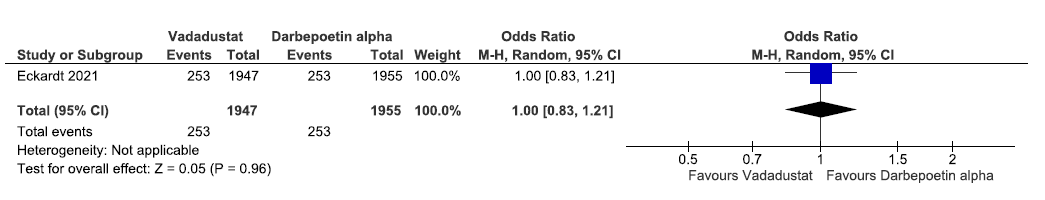
- Forest plot for vadadustat versus darbepoetin alpha on all-cause mortality up to 116 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method.
Effect of HIF-PHI on the need for oral/intravenous iron supplementation
We found nine studies reporting effect of HIF-PHIs on the need for iron supplementation as compared to ESAs.
Effect of daprodustat versus ESAs (rhEPO/darbepoetin alpha/epoetin alpha) on the need for oral iron supplementation up to 52 weeks
One study reported the need for oral iron supplementation up to 52 weeks in daprodustat as compared to ESAs. Daprodustat reduced the need for oral iron supplementation up to 52 weeks as compared to ESAs [OR: 0.91 (95% CI 0.55–1.52); p = 0.73; 267 participants; very low certainty evidence].18 The forest plot is shown in Figure 17.
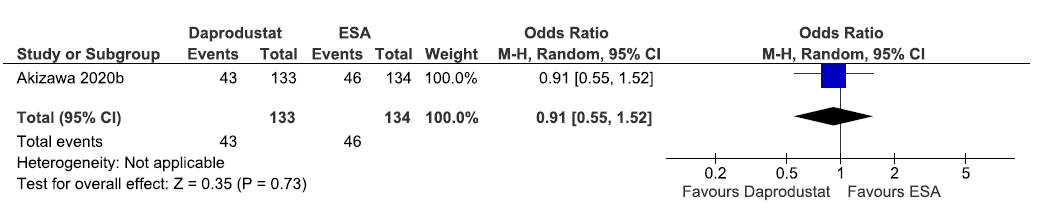
- Forest plot for daprodustat versus ESA on the need for oral iron supplementation up to 52 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents.
Effect of daprodustat versus ESAs (rhEPO/darbepoetin alpha/epoetin alpha) on the need for intravenous (IV) iron supplementation up to 52 weeks
Two studies reported the need for IV iron supplementation up to 52 weeks in daprodustat as compared to ESAs. The pooled results reported daprodustat reduced the need for IV iron supplementation up to 52 weeks as compared to ESAs [OR: 0.77 (95% CI 0.53–1.13); p = 0.18; two studies; 674 participants; moderate certainty evidence].18,22 The forest plot is shown in Figure 18.
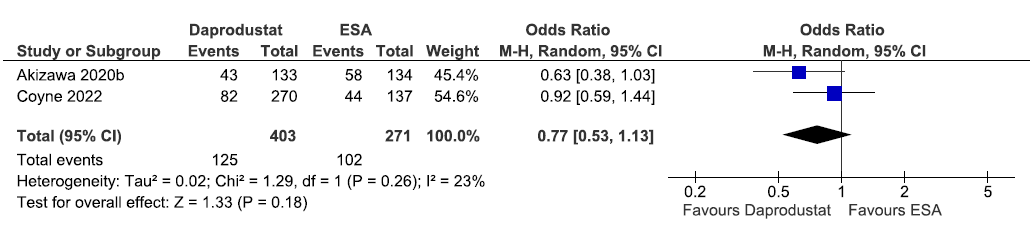
- Forest plot for daprodustat versus ESA on the need for intravenous iron supplementation up to 52 weeks. CI: Confidence intervals, IV: Inverse variance, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method, df: degrees of freedom.
Effect of enarodustat versus darbepoetin alpha on the need for oral iron supplementation up to 24 weeks
One study reported the need for oral iron supplementation up to 24 weeks in enarodustat as compared to darbepoetin alpha. The results reported that enarodustat increased the need for oral iron supplementation up to 24 weeks as compared to darbepoetin alpha [OR: 1.40 (95% CI 0.76–2.56); p = 0.28; 172 participants; very low certainty evidence].16 The forest plot is shown in Figure 19.
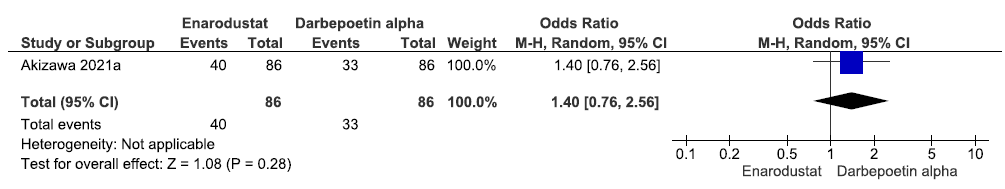
- Forest plot for enarodustat versus darbepoetin alpha on the need for oral iron supplementation up to 24 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method.
Effect of molidustat versus ESA on the need for oral iron supplementation up to 52 weeks
One study reported the need for oral iron supplementation up to 52 weeks in molidustat as compared to ESAs. The results reported molidustat increased the need for oral iron supplementation up to 52 weeks as compared to ESAs [OR: 3.45 (95% CI 0.99–12.05); p = 0.05; 229 participants; very low certainty evidence].17 The forest plot is shown in Figure 20.
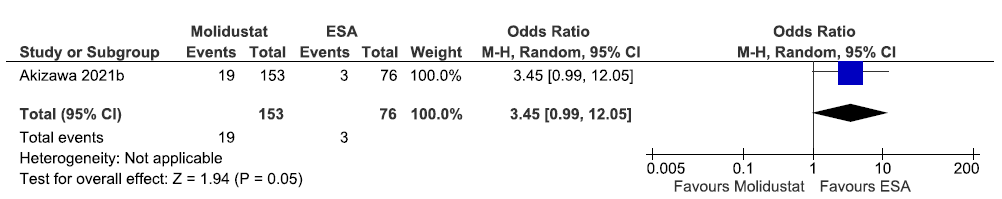
- Forest plot for molidustat versus ESA on the need for oral iron supplementation up to 52 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents.
Effect of molidustat versus ESA on the need for IV iron supplementation up to 52 weeks
One study reported the need for IV iron supplementation up to 52 weeks in molidustat as compared to ESAs. The results reported molidustat decreased the need for IV iron supplementation up to 52 weeks as compared to ESAs [OR: 0.96 (95% CI 0.54–1.69); p = 0.88; 229 participants; very low certainty evidence].17 The forest plot is shown in Figure 21.
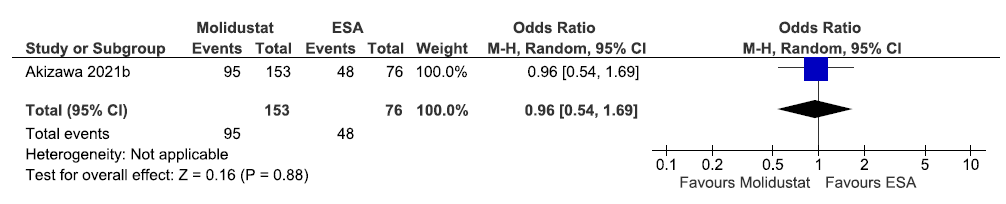
- Forest plot for molidustat versus ESA on the need for IV iron supplementation up to 52 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents, IV: Inverse variance.
Effect of roxadustat versus ESA on the need for iron supplementation up to 6–52 weeks
Three studies reported the need for iron supplementation up to 6–52 weeks in roxadustat as compared to ESAs. The pooled results reported roxadustat decreased the need for iron supplementation up to 6–52 weeks as compared to ESAs [OR: 0.57 (95% CI 0.16–2.05); p = 0.39; three studies; 1215 participants; very low certainty evidence].21,31,32 The forest plot is shown in Figure 22.
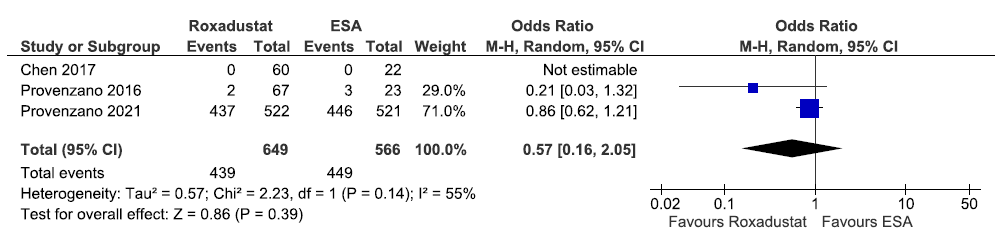
- Forest plot for roxadustat versus ESA on the need for iron supplementation up to 6–52 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents, df: degrees of freedom.
Effect of roxadustat versus ESA on the need for iron supplementation up to 52–208 weeks
Two studies reported the need for iron supplementation up to 52–208 weeks in roxadustat as compared to ESAs. The pooled results reported roxadustat decreased the need for iron supplementation up to 52–208 weeks as compared to ESAs [OR: 0.56 (95% CI 0.13–2.46); p = 0.45; two studies; 2940 participants; very low certainty evidence].23,25 The forest plot is shown in Figure 23.
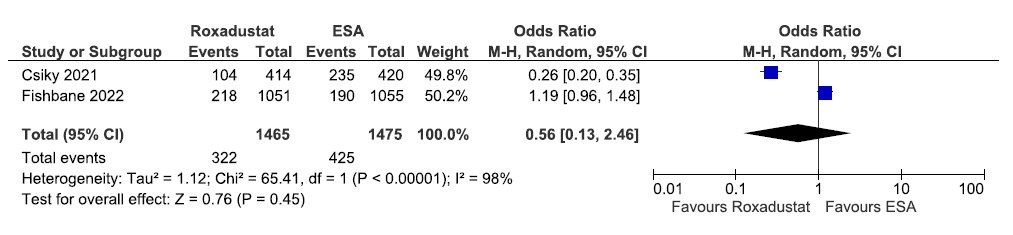
- Forest plot for roxadustat versus ESA on the need for iron supplementation up to 52–208 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents, df: degrees of freedom.
Effect of HIF-PHI on the need for ESA
We found five studies reporting the effect of HIF-PHIs on the need for ESA as compared to ESAs.
Effect of molidustat versus ESAs (rhEPO/darbepoetin alpha/epoetin alpha) on the need for ESA up to 52 weeks
One study reported the need for ESA up to 52 weeks in molidustat as compared to ESAs. The results reported molidustat increases the need for an EPO-stimulating agent up to 52 weeks as compared to ESAs [OR: 8.15 (95% CI 1.06–62.93); p = 0.04; 229 participants; very low certainty evidence].17 The forest plot is shown in Figure 24.
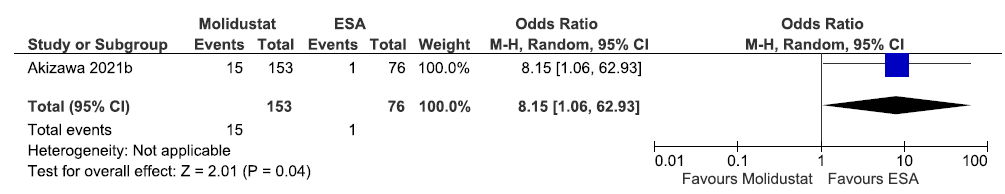
- Forest plot for molidustat versus ESAs on the need for ESA up to 52 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents.
Effect of roxadustat versus ESA on the need for ESA up to 6–52 weeks
Two studies reported the need for ESA up to 6–52 weeks in roxadustat as compared to ESAs. The pooled results reported roxadustat increased the need for ESA up to 6–52 weeks as compared to ESAs [OR: 13.38 (95% CI 0.75–238.31); p = 0.08; two studies; 916 participants; very low certainty evidence].21,23 The forest plot is shown in Figure 25.
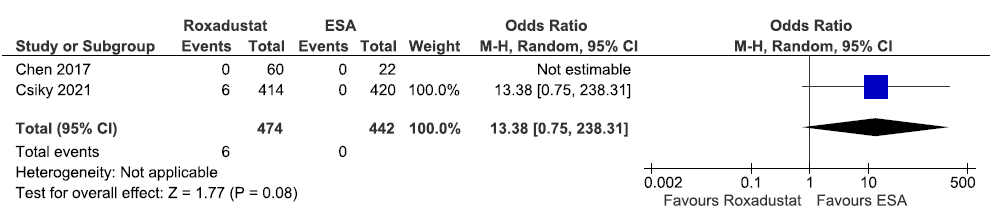
- Forest plot for roxadustat versus ESA on the need for Erythropoietin Stimulating Agent up to 6-52 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents.
Effect of roxadustat versus ESA on the need for ESA up to 208 weeks
One study reported the need for ESA up to 208 weeks in roxadustat as compared to ESAs. Roxadustat increased the need for ESA up to 208 weeks as compared to ESAs [OR: 20.29 (95% CI 4.89–84.25); p < 0.0001; 2106 participants; very low certainty evidence].25 The forest plot is shown in Figure 26.
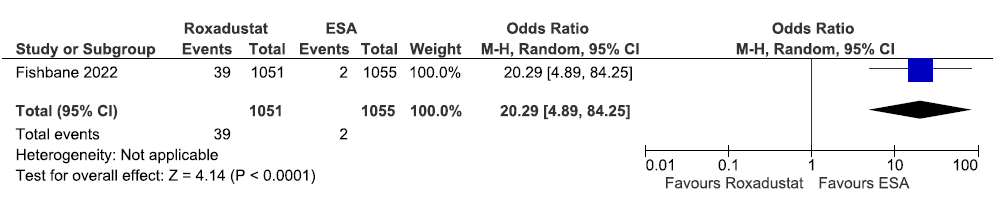
- Forest plot for roxadustat versus ESA on the need for ESA up to 208 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents.
Effect of vadadustat versus darbepoetin alpha on the need for ESA in the incident dialysis group up to 116 weeks
One study reported the need for ESA in the incident dialysis group up to 116 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat increased the need for ESA in the incident dialysis group up to 116 weeks as compared to ESAs [OR: 1.75 (95% CI 0.83–3.71); p = 0.14; 265 participants; very low certainty evidence].24 The forest plot is shown in Figure 27.
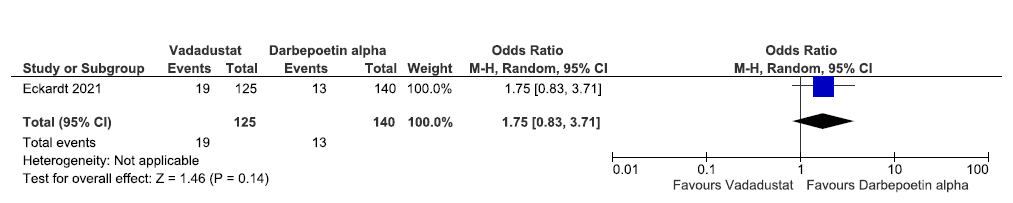
- Forest plot for vadadustat versus darbepoetin alpha on the need for ESA in incident dialysis up to 116 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents.
Effect of vadadustat versus darbepoetin alpha on the need for ESA in the prevalent dialysis group up to 116 weeks
One study reported the need for ESA in the prevalent dialysis group up to 116 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat increased the need for ESA in the incident dialysis group up to 116 weeks as compared to ESAs [OR: 1.25 (95% CI 1.03–1.51); p = 0.02; 2792 participants; low certainty evidence].24 The forest plot is shown in Figure 28.
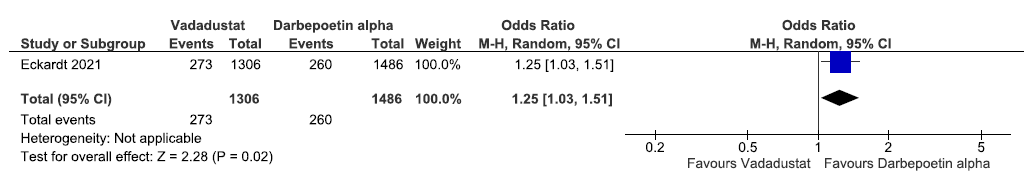
- Forest plot for vadadustat versus Darbepoetin alpha on the need for ESA in prevalent dialysis up to 116 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents.
Effect of HIF-PHIs on health-related quality of life
We found two studies reporting the effect of HIF-PHIs on health-related quality of life (HRQoL) as compared to ESAs.
Effect of desidustat versus epoetin alfa on the QoL assessed by SF-36 up to 24 weeks
One study reported QoL assessed by SF-36 up to 24 weeks in desidustat as compared to epoetin alpha. The study reported desidustat worsens the QoL assessed by SF-36 up to 24 weeks as compared to epoetin alfa [MD: -49.73 (95% CI -144.53–45.07); p = 0.30; 346 participants; very low certainty evidence].26 The forest plot is shown in Figure 29.
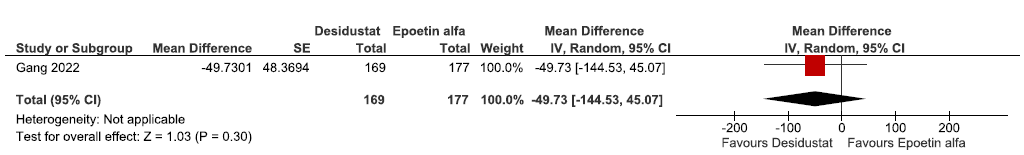
- Forest plot for desidustat versus epoetin alfa on the QoL assessed by SF-36 up to 24 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents, SE: Standard error, QoL: Quality of Life.
Effect of roxadustat versus ESA on the QoL assessed by EQ-5D-5L VAS up to 12–28 weeks
One study reported QoL assessed by EQ-5D-5L VAS up to 12–28 weeks in roxadustat as compared to ESAs. Roxadustat improved the QoL assessed by EQ-5D-5L VAS up to 12–28 weeks as compared to ESAs [MD: 1.42 (95% CI -1.21–4.04); p = 0.29; 783 participants; very low certainty evidence].23 The forest plot is shown in Figure 30.
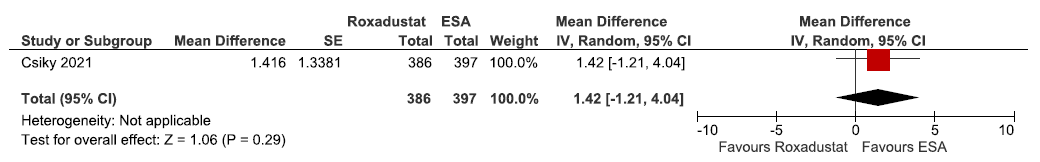
- Forest plot for roxadustat versus ESA on the QoL assessed by EQ-5D-5L VAS up to 12–28 weeks. CI: Confidence intervals, IV: Inverse variance, ESA: Eythropoiesis-stimulating agents, SE: Standard error, QoL: Quality of Life.
Effect of HIF-PHI on fatigue
We found one study reporting the effect of HIF-PHIs on fatigue as compared to ESAs.
Effect of roxadustat versus ESA on fatigue measured by FACT with a total score at 28 weeks
One study reported fatigue measured by FACT with a total score at 28 weeks in roxadustat as compared to ESAs. Roxadustat increased fatigue measured by FACT with a total score at 28 weeks as compared to ESAs [MD: 2.41 (95% CI -1.68–6.51); p = 0.25; 783 participants; very low certainty evidence].23 The forest plot is shown in Figure 31.
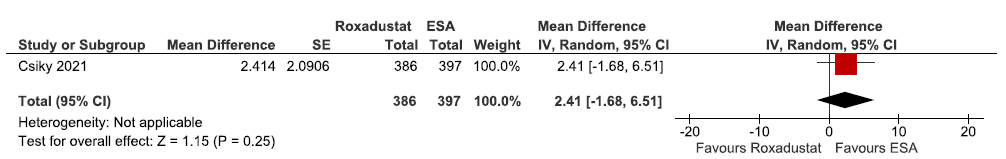
- Forest plot for roxadustat versus ESA on fatigue measured by FACT with a total score at 28 weeks. CI: Confidence intervals, IV: Inverse variance, ESA: Eythropoiesis-stimulating agents, SE: Standard error, FACT: Functional Assessment of Cancer Therapy (measure of fatigue).
Effect of HIF-PHI on incidences of MACE and MACE plus
We found six studies reporting the effect of HIF-PHIs on incidences of MACE and MACE plus as compared to ESAs.
Effect of daprodustat vs darbepoetin alpha on the incidences of MACE up to 52 weeks
Three studies reported incidences of MACE up to 52 weeks in daprodustat as compared to darbepoetin alpha. The pooled results reported that daprodustat decreased the incidence of MACE up to 52 weeks as compared to darbepoetin alpha [OR: 0.95 (95% CI 0.82–1.11); p = 0.55; three studies; 3691 participants; low certainty evidence].22,33,34 The forest plot is shown in Figure 32.
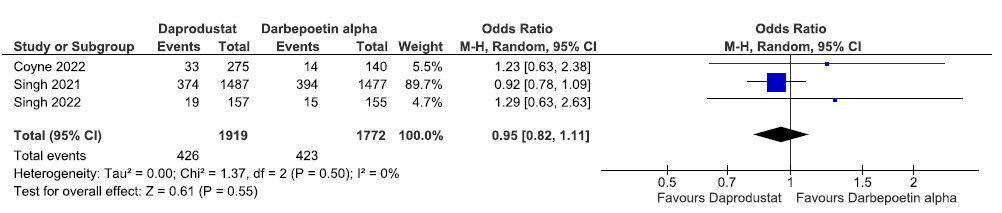
- Forest plot for daprodustat versus darbepoetin alpha on the incidence of MACE up to 52 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents, SE: Standard error, df: degrees of freedom, MACE: Major adverse cardiovascular events.
Effect of molidustat versus ESA on the incidence of MACE up to 52 weeks
One study reported the incidence of MACE up to 52 weeks in molidustat as compared to ESAs. Molidustat increased the incidence of MACE up to 52 weeks as compared to ESAs [OR: 1.25 (95% CI 0.24–6.60); p = 0.79; 229 participants; very low certainty evidence].17 The forest plot is shown in Figure 33.
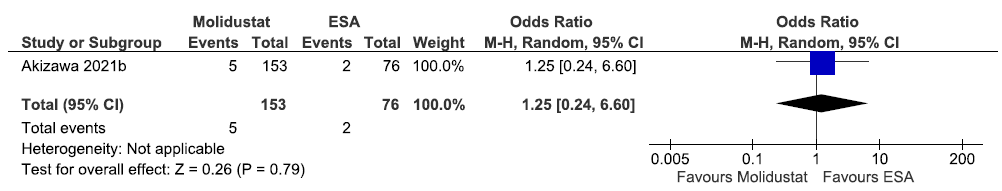
- Forest plot for molidustat versus ESA on the incidence of MACE up to 52 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents, MACE: Major adverse cardiovascular events.
Effect of roxadustat versus ESA on the incidence of MACE up to six weeks
One study reported the incidence of MACE up to six weeks in roxadustat as compared to ESAs. There were none who experienced MACE up to six weeks to determine whether roxadustat made a difference as compared to ESA (OR: not estimable; 96 participants; very low certainty evidence).21 The forest plot is shown in Figure 34.
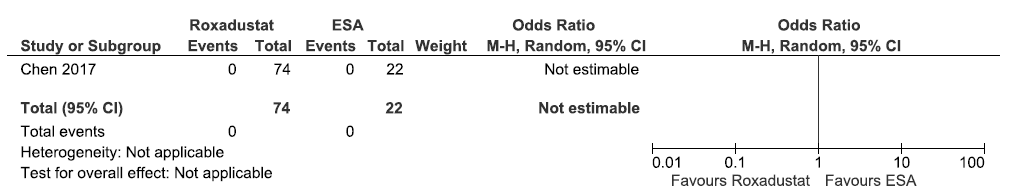
- Forest plot for roxadustat versus ESA on the incidence of MACE up to 6 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents, MACE: Major adverse cardiovascular events.
Effect of vadadustat versus darbepoetin alpha on the incidence of MACE up to 116 weeks
One study reported incidence of MACE up to 116 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat decreased incidence of MACE up to 116 weeks as compared to darbepoetin alpha [OR: 0.93 (95% CI 0.79–1.10); p = 0.40; 3902 participants; very low certainty evidence].24 The forest plot is shown in Figure 35.
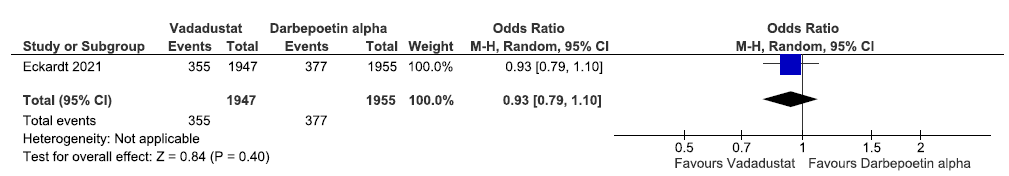
- Forest plot for vadadustat versus darbepoetin alpha on the incidence of MACE up to 116 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents, MACE: Major adverse cardiovascular events.
Effect of vadadustat versus darbepoetin alpha on the incidence of MACE plus up to 116 weeks
One study reported incidence of MACE plus up to 116 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat decreased incidence of MACE plus up to 116 weeks as compared to darbepoetin alpha [OR: 0.92 (95% CI 0.79–1.07); p = 0.30; 3902 participants; very low certainty evidence].24 The forest plot is shown in Figure 36.
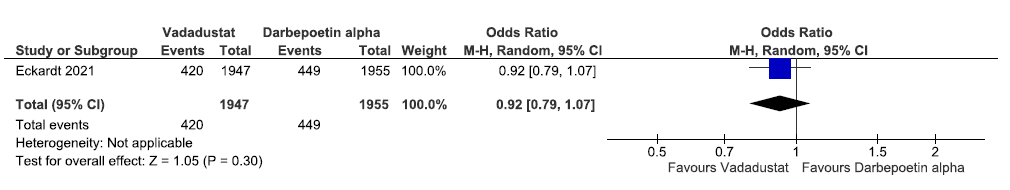
- Forest plot for vadadustat versus darbepoetin alpha on the incidence of MACE plus up to 116 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, ESA: Eythropoiesis-stimulating agents, MACE: Major adverse cardiovascular events.
Effect of HIF-PHI on Treatment Emergent Adverse Events
We found 18 studies reporting the effect of HIF-PHIs on treatment emergent adverse events (TEAEs) as compared to ESAs.
Effect of desidustat versus epoetin alfa on the TEAEs up to 26 weeks
One study reported TEAEs up to 26 weeks in desidustat as compared to epoetin alpha. The study reported that desidustat increased TEAEs up to 26 weeks as compared to epoetin alfa [OR: 1.06 (95% CI 0.72–1.58); p = 0.76; 392 participants; very low certainty evidence].26 The forest plot is shown in Figure 37.
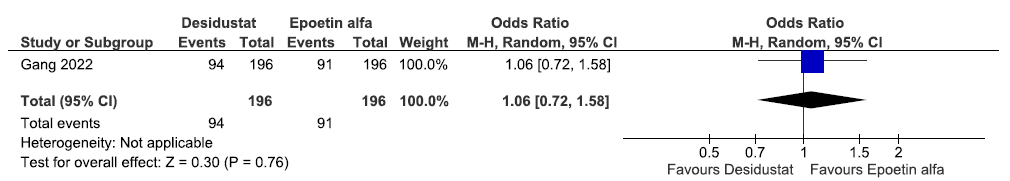
- Forest plot for desidustat versus epoetin alfa on the TEAEs up to 26 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method, TEAE: Treatment Emergent Adverse Events.
Effect of daprodustat versus ESAs (rhEPO/darbepoetin alpha/epoetin alpha) on adverse events up to 52 weeks
Four studies reported adverse events up to 52 weeks in daprodustat as compared to ESAs. The pooled results reported daprodustat had little or no difference on adverse events up to 52 weeks as compared to ESAs [OR: 1.05 (95% CI 0.73–1.50); p = 0.80; four studies; 3945 participants; low certainty evidence].18,22,33,34 The forest plot is shown in Figure 38.
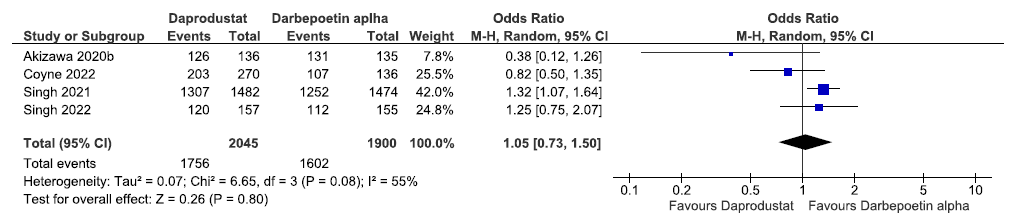
- Forest plot for daprodustat versus ESAs on the adverse events up to 52 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method, df: degrees of freedom.
Effect of enarodustat versus darbepoetin alpha on adverse events up to 26 weeks
One study reported adverse event up to 26 weeks in enarodustat as compared to darbepoetin alpha. Enarodustat increased adverse events up to 26 weeks as compared to darbepoetin alpha [OR: 1.34 (95% CI 0.57–3.15); p = 0.50; 173 participants; very low certainty evidence].16 The forest plot is shown in Figure 39.
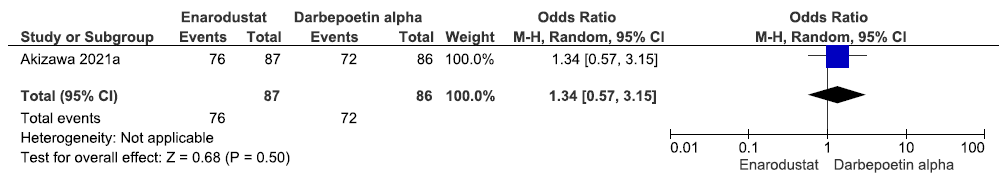
- Forest plot for enarodustat versus darbepoetin alpha on the adverse events up to 26 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method.
Effect of molidustat versus ESAs on TEAEs up to 52 weeks.
Two studies reported TEAE up to 52 weeks in molidustat as compared to ESAs. The pooled results reported that molidustat increased treatment emergent adverse up to 52 weeks as compared to ESAs [OR: 1.24 (95% CI 0.62–2.45); p = 0.54; two studies; 428 participants; very low certainty evidence].17,29 The forest plot is shown in Figure 40.
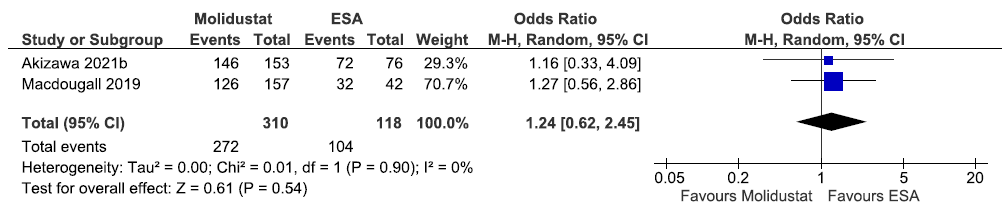
- Forest plot for molidustat versus ESA on the TEAE up to 52 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method, df: degrees of freedom, TEAE: Treatment emergent adverse events.
Effect of roxadustat versus ESA on TEAEs up to 6–52 weeks
Six studies reported TEAEs up to 6–52 weeks in roxadustat as compared to ESAs. The pooled results reported that roxadustat increased TEAEs up to 6–52 weeks as compared to ESAs [OR: 1.45 (95% CI 1.08–1.96); p = 0.01; six studies; 1715 participants; moderate certainty evidence].15,19–21,28,31 The forest plot is shown in Figure 41.
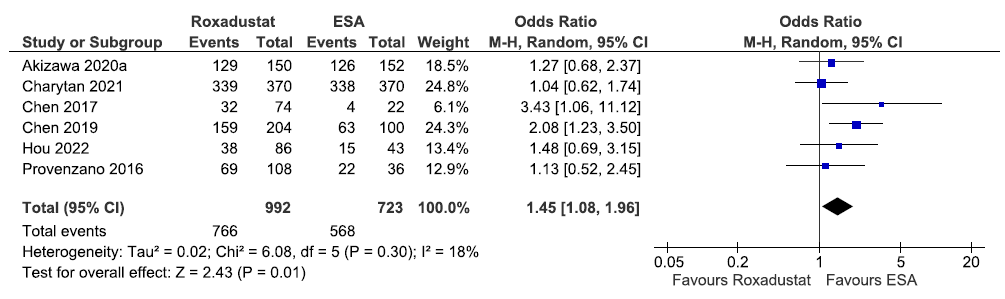
- Forest plot for roxadustat versus ESA on the TEAEs up to 6–52 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method, df: degrees of freedom, TEAE: Treatment emergent adverse events.
Effect of roxadustat versus ESA on TEAEs up to 108–209 weeks
Two studies reported TEAEs up to 108–209 weeks in roxadustat as compared to ESAs. The pooled results reported that roxadustat have little or no difference on TEAEs up to 108–209 weeks as compared to ESAs [OR: 1.05 (95% CI 0.85–1.28); p = 0.66; two studies; 2935 participants; very low certainty evidence].23,25 The forest plot is shown in Figure 42.
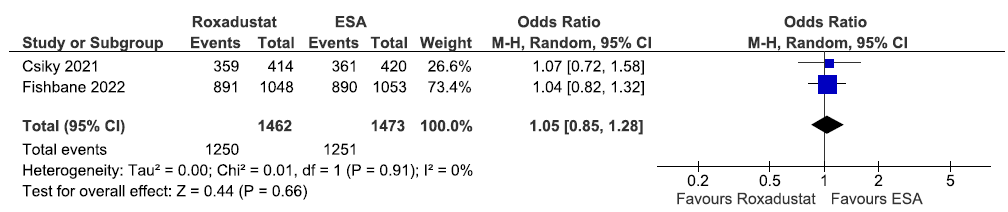
- Forest plot for roxadustat versus ESA on the TEAEs up to 108–209 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method, df: degrees of freedom, TEAE: Treatment emergent adverse events.
Effect of vadadustat versus darbepoetin alpha on adverse events in the incident dialysis group up to 116 weeks
One study reported adverse event in the incident dialysis group up to 116 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat decreased adverse event in the incident dialysis group up to 116 weeks as compared to ESAs [OR: 0.88)95% CI 0.50–1.55); p = 0.66; 365 participants; very low certainty evidence].24 The forest plot is shown in Figure 43.
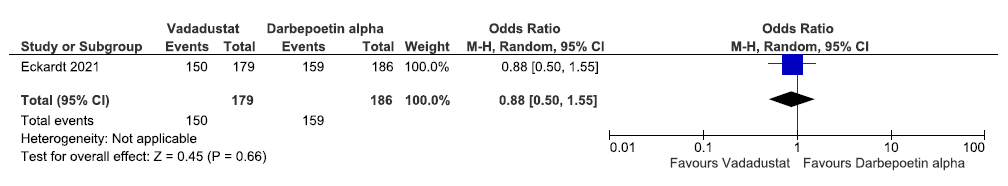
- Forest plot for vadadustat versus darbepoetin alpha on the adverse events in incident dialysis up to 116 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method.
Effect of vadadustat versus darbepoetin alpha on adverse events in the prevalent group up to 116 weeks
One study reported adverse event in the prevalent dialysis group up to 116 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat decreased adverse event in the prevalent dialysis group up to 116 weeks as compared to ESAs [OR: 0.91 (95% CI 0.74–1.12); p = 0.36; 3537 participants; very low certainty evidence].24 The forest plot is shown in Figure 44.
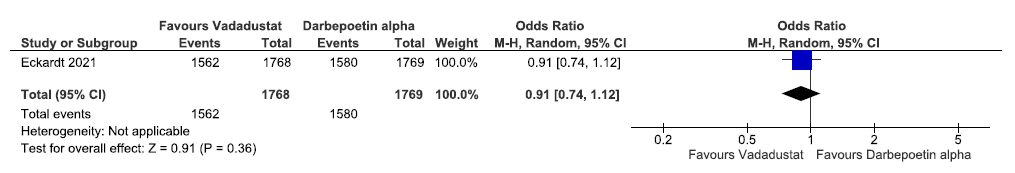
- Forest plot for vadadustat versus darbepoetin alpha on the adverse events in prevalent dialysis up to 116 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method.
Effect of vadadustat versus darbepoetin alpha on adverse events up to 52 weeks
One study reported on adverse events up to 52 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat decreased adverse event up to 52 weeks as compared to darbepoetin alpha [OR: 0.37 (95% CI 0.10–1.40); p = 0.14; 323 participants; very low certainty evidence].30 The forest plot is shown in Figure 45.
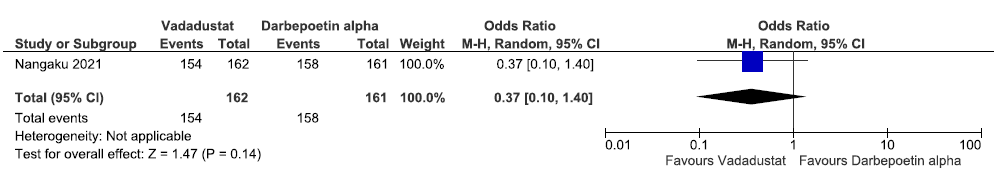
- Forest plot for vadadustat versus darbepoetin alpha on the adverse events up to 52 weeks. CI: Confidence intervals, M-H: Mantel-Haenszel method.
Effect of HIF-PHI on requirement of Blood Transfusion
We found six studies reporting effect of HIF-PHIs on patients requiring blood transfusion as compared to ESAs.
Effect of daprodustat versus ESAs (rhEPO/darbepoetin alpha/epoetin alpha) on requirement of blood transfusion up to 52 weeks
One study reported patients requiring blood transfusion up to 52 weeks in daprodustat as compared to ESAs. Daprodustat decreased patients requiring blood transfusion up to 52 weeks as compared to ESAs [OR: 0.83 (95% CI 0.69–1.01); p = 0.07; 2964 participants; low certainty evidence].33 The forest plot is shown in Figure 46.
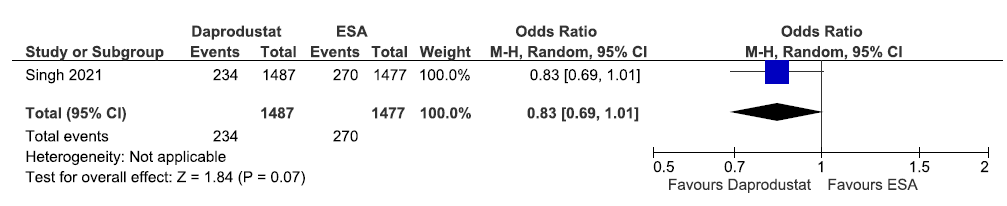
- Forest plot for daprodustat versus ESAs on the patients requiring blood transfusion up to 52 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method.
Effect of molidustat versus ESAs on requirement of blood transfusion up to 20 weeks
One study reported patients requiring blood transfusion up to 20 weeks in molidustat as compared to ESAs. Molidustat increased patients requiring blood transfusion up to 20 weeks as compared to ESAs [OR: 1.51 (95% CI 0.32–7.08); p = 0.60; 199 participants; very low certainty evidence].29 The forest plot is shown in Figure 47.
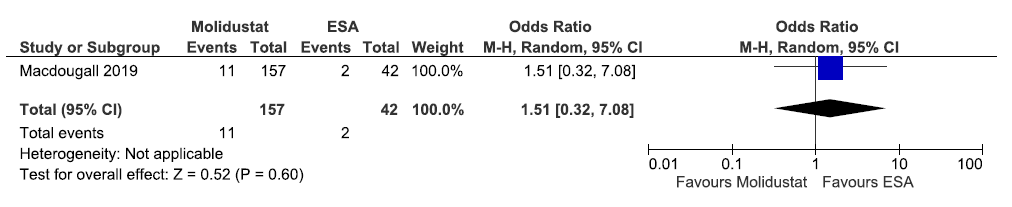
- Forest plot for molidustat versus ESA on the patients requiring blood transfusion up to 20 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method.
Effect of roxadustat versus ESA on requirement of blood transfusion up to 6–52 weeks
Two studies reported patients requiring blood transfusion up to 6–52 weeks in roxadustat as compared to ESAs. The pooled results reported roxadustat reduced patients requiring blood transfusion up to 6–52 weeks as compared to ESAs [OR: 0.52 (95% CI 0.35–0.78); p = 0.001; two studies; 821 participants; very low certainty evidence].19,21 The forest plot is shown in Figure 48.
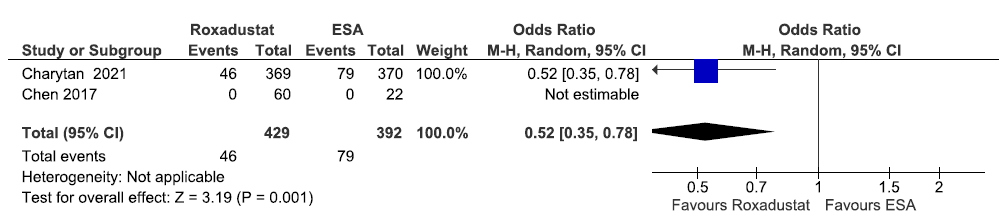
- Forest plot for roxadustat versus ESA on the patients requiring blood transfusion up to 6–52 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method.
Effect of roxadustat versus ESA on requirement of blood transfusion up to 58–108 weeks
Two studies reported patients requiring blood transfusion up to 58–108 weeks in roxadustat as compared to ESAs. The pooled results reported roxadustat reduced patients requiring blood transfusion up to 58–108 weeks as compared to ESAs [OR: 0.88 (95% CI 0.53–1.44); p = 0.60; two studies; 1869 participants; very low certainty evidence].23,32 The forest plot is shown in Figure 49.
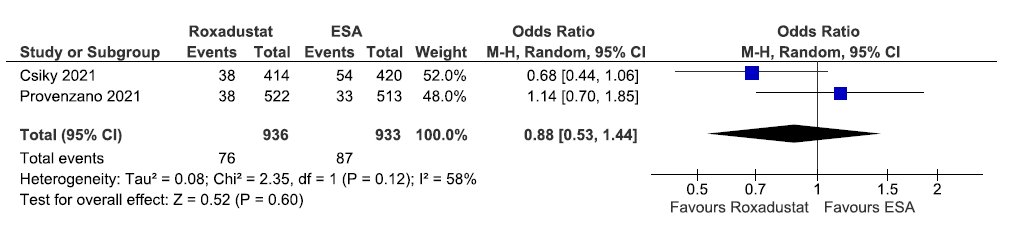
- Forest plot for roxadustat versus ESA on the patients requiring blood transfusion up to 58–108 weeks. CI: Confidence intervals, ESA: Eythropoiesis-stimulating agents, M-H: Mantel-Haenszel method, df: degrees of freedom.
Discussion
Our systematic review identified 20 trials on HIF-PHIs for treatment of anemia in DD-CKD patients. The effect estimates as well as the certainty of evidence varied across outcomes for different HIF-PHIs. Risks of bias in the included studies were often high or unclear. The lack of high certainty evidence across outcomes and across HIF-PHI molecules is a constant aspect.
HIF-PHIs have been termed as a new breakthrough approach for managing anemia in patients with CKD, preferably because of its capability to enhance hematological outcomes.35,36 Other alternatives however like ESAs pose challenges in patients on dialysis like hyporesponsiveness in elderly patients, nonfatal myocardial infarction, congestive heart failure, and cerebral apoplexy.37,38 Therefore, these reasons make it feasible to use HIF-PHIs in DD patients with CKD for managing renal anemia. Other potential advantages of HIF-PHIs over ESAs include:
Increasing hemoglobin without the risk of raising in blood pressure
Reducing the need for iron replacement therapy
Administered orally (unlike ESA) and avoiding the need for injection with good compliance.
The results of this review can inform clinical practitioners to take decisions wisely and choose appropriate treatment for the patients and policy. The evidence would also aid in prioritizing funding and conducting high-quality clinical trials to provide evidence on the efficacy and safety of HIF-PHIs.
Among different HIF-PHIs, desidustat, enarodustat, molidustat, and vadadustat showed little to no difference or small benefit. Daprodustat had substantial net benefits as compared to ESAs while roxadustat as an alternative to ESAs did more harm than benefit. Daprodustat favored and decreased the need for intravenous iron supplementation as compared ESAs (moderate certainty evidence). Roxadustat increased TEAEs up to 6–52 weeks than ESAs (moderate certainty evidence on GRADE). Roxadustat significantly raised the hemoglobin levels from baseline up to 6–52 weeks than ESAs (low certainty evidence). While desidustat and daprodustat showed an equivalent effect as that of ESAs on change in the hemoglobin levels from baseline (very low and low certainty evidence, respectively), existing evidence was of very low certainty for majority of the outcomes. The evidence was rated very low mainly due to serious imprecision in effect estimates. This was due to the limited number of trials conducted on individual HIF-PHI agents with inadequate number of participants.
We report an equivalent effect of HIF-PHIs and ESAs on change in the hemoglobin levels. However, HIF-PHIs have uncertain effects on MACE, fatigue, and all-cause mortality. These findings are consistent with the existing literature.39 Our review also reported that roxadustat significantly favored increased hemoglobin levels from ESAs. The review findings are also generally in line with other systematic reviews.40–42
The review was conducted as per the protocol which was registered a priori. We used robust methodologies with comprehensive search strategies. Screening and data extraction process were performed independently by at least two authors. We acknowledge a limitation that the certainty of evidence using GRADE was initially determined by a single reviewer and cross-checked by another reviewer and not independently, as is the best practice.
Our meta-analysis provided evidence on the use of HIF-PHIs as an alternative to ESAs for DD-CKD patients at a molecule level and for clinically important outcomes.
Financial support and sponsorship
The systematic review was done to support a guideline development work. The George Institute for Global Health India received an unrestricted institutional grant from Zydus Lifesciences Ltd for the guideline development. The funder has no role in any part of the systematic review, including the decision to conduct it, methodology, or publish it.
Conflicts of interest
There are no conflicts of interest.
References
- Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet. 2020;395:709-33.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Anaemia of chronic kidney disease: An under-recognized and under-treated problem. Nephrol Dial Transplant. 2002;17(Suppl 11):44-6.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety of roxadustat for anemia in patients with chronic kidney disease: A meta-analysis. Nephrol Dial Transplant. 2021;36:1603-15.
- [CrossRef] [PubMed] [Google Scholar]
- Iron therapy in chronic kidney disease: Days of future past. Int J Mol Sci. 2021;22:1008.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A new approach to treating renal anaemia. Nat Rev Nephrol. 2019;15:731-2.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy and safety of hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI) on anemia in non-dialysis-dependent chronic kidney disease (NDD-CKD): A systematic review and meta-analysis. Int Urol Nephrol. 2021;53:1139-47.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Recommendations by the Asian Pacific society of nephrology (APSN) on the appropriate use of HIF-PH inhibitors. Nephrology (Carlton). 2021;26:105-18.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- FibroGen. FibroGen reports third quarter 2021 financial results. Global newswire; 2021. 9th November 2021. 20th January, 2022. Available from: https://www.globenewswire.com/en/news-release/2021/11/09/2330835/33525/en/FibroGen-Reports-Third-Quarter-2021-Financial-Results.html [Last accessed 2022 January 20].
- Desidustat: First Approval. Drugs. 2022;82:1207-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors for Treatment of Anemia in Chronic Kidney Disease: Guidelines for South Asia. Indian J Nephrol. 2025;35:129-67.
- [CrossRef] [Google Scholar]
- Rayyan—A web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cochrane handbook for systematic reviews of interventions. Chichester (UK): John Wiley & Sons; 2019.
- The GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group; 2013.
- Phase 3, randomized, double-blind, active-comparator (Darbepoetin Alfa) study of oral roxadustat in CKD Patients with anemia on hemodialysis in Japan. J Am Soc Nephrol. 2020;31:1628-39.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A Phase 3 Study of Enarodustat (JTZ-951) in Japanese hemodialysis patients for treatment of anemia in chronic kidney disease: SYMPHONY HD study. Kidney Dis (Basel). 2021;7:494-502.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Molidustat for Japanese patients with renal anemia receiving dialysis. Kidney Int Rep. 2021;6:2604-16.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy and safety of daprodustat compared with darbepoetin alfa in Japanese hemodialysis patients with anemia: A randomized, double-blind, phase 3 trial. Clin J Am Soc Nephrol. 2020;15:1155-65.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A randomized trial of roxadustat in anemia of kidney failure: SIERRAS Study. Kidney Int Rep. 2021;6:1829-39.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med. 2019;381:1011-22.
- [CrossRef] [PubMed] [Google Scholar]
- Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017;32:1373-86.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Three times weekly dosing of daprodustat versus conventional epoetin for treatment of anemia in hemodialysis patients: ASCEND-TD: A phase 3 randomized, double-blind, noninferiority trial. Clin J Am Soc Nephrol. 2022;17:1325-36.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Roxadustat for the maintenance treatment of anemia in patients with end-stage kidney disease on stable dialysis: A European phase 3, randomized, open-label, active-controlled study (PYRENEES) Adv Ther. 2021;38:5361-80.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Safety and efficacy of vadadustat for anemia in patients undergoing dialysis. N Engl J Med. 2021;384:1601-12.
- [CrossRef] [PubMed] [Google Scholar]
- Roxadustat versus epoetin alfa for treating anemia in patients with chronic kidney disease on dialysis: Results from the randomized phase 3 ROCKIES study. J Am Soc Nephrol. 2022;33:850-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Desidustat in anemia due to dialysis-dependent chronic kidney disease: A phase 3 study (DREAM-D) Am J Nephrol. 2022;53:343-51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor gsk1278863 for treatment of anemia. J Am Soc Nephrol. 2016;27:1234-44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Roxadustat treatment for anemia in peritoneal dialysis patients: A randomized controlled trial. J Formos Med Assoc. 2022;121:529-38.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of molidustat in the treatment of anemia in CKD. Clin J Am Soc Nephrol. 2019;14:28-39.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy and safety of vadadustat compared with darbepoetin alfa in Japanese anemic patients on hemodialysis: A Phase 3, multicenter, randomized, double-blind study. Nephrol Dial Transplant. 2021;36:1731-41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: A phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis. 2016;67:912-24.
- [CrossRef] [PubMed] [Google Scholar]
- Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrol Dial Transplant. 2021;36:1717-30.
- [CrossRef] [PubMed] [Google Scholar]
- Daprodustat for the treatment of anemia in patients undergoing dialysis. N Engl J Med. 2021;385:2325-35.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of daprodustat for treatment of anemia of chronic kidney disease in incident dialysis patients: A randomized clinical trial. JAMA Intern Med. 2022;182:592-602.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prolyl hydroxylase inhibitors: A breakthrough in the therapy of anemia associated with chronic diseases. J Med Chem. 2018;61:6964-82.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia-inducible factor-prolyl hydroxylase inhibitors in the treatment of anemia of chronic kidney disease. Kidney Int Suppl (2011). 2021;11:8-25.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Is HIF-PHI the answer to tackle ESA hyporesponsiveness in the elderly? Kidney and Dialysis. 2022;2:446-453. Available from: https://doi.org/10.3390/kidneydial2030040
- [CrossRef] [Google Scholar]
- Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085-98.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia-inducible factor stabilisers for the anaemia of chronic kidney disease. Cochrane Database Syst Rev. 2022;8:CD013751.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The efficacy and safety of roxadustat treatment for anemia in patients with kidney disease: A meta-analysis and systematic review. Int Urol Nephrol. 2021;53:985-97.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Safety and efficacy of roxadustat for anemia in patients with chronic kidney disease: A meta-analysis and trial sequential analysis. Front Med (Lausanne). 2021;8:724456.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of hypoxia-inducible factor-prolyl hydroxylase inhibitors on anemia in patients with CKD: A meta-analysis of randomized controlled trials including 2804 patients. Ren Fail. 2020;42:912-25.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








