Translate this page into:
Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors for Anemia in Non-Dialysis Dependent Chronic Kidney Disease: Systematic Review and Meta-Analysis of Randomized Controlled Trials
Corresponding author: Soumyadeep Bhaumik, Meta-Research and Evidence Synthesis Unit, The George Institute for Global Health, New Delhi, India. E-mail: sbhaumik@georgeinstitute.org
-
Received: ,
Accepted: ,
How to cite this article: Tyagi J, Kaur M, Moola S, Ramachandran R, Meena P, Bajpai D, et al. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors for Anemia in Non-Dialysis Dependent Chronic Kidney Disease: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Indian J Nephrol. 2025;35:217-33. doi: 10.25259/ijn_382_23
Abstract
Background
Hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs) is a new therapy option for anemia in chronic kidney disease (CKD) patients. We aimed to evaluate evidence from randomized controlled trials (RCTs) on HIF-PHIs for anemia in non-dialysis dependent (NDD)-CKD patients.
Materials and Methods
We searched three electronic databases (PubMed, CINAHL, Cochrane Central Register of Controlled Trials databases), trial registries, and manually screened reference list. Two authors independently conducted screening, data extraction, and assessed risk of bias. We used RevMan 5.3 for meta-analysis using standard methods. Certainty of evidence was assessed by Grading of Recommendations, Assessment, Development, and Evaluations.
Results
We included 12 RCTs involving 8611 patients with anemia of kidney disease. The studies included roxadustat (n = 2), daprodustat (n = 3), molidustat (n = 3), vadadustat (n = 2), enarodustat (n = 1), and desidustat (n = 1). Desidustat and daprodustat reported no difference in the hemoglobin levels from baseline up to 24–52 weeks as compared to darbepoetin alpha [Mean Difference (MD): 0.09 g/dL (CI 95% 0.15–0.33); p = 0.46; 529 participants; low certainty evidence; and MD: 0.08 g/dL (CI 95% 0.08–0.08); p < 0.00001; two studies; 4089 participants; low certainty evidence, respectively]. Broadly, HIF-PHI molecules exhibited little difference when compared to other alternatives like erythropoietin stimulating agents (ESAs), but the evidence is not of high certainty.
Conclusion
Our meta-analysis provides evidence on the use of HIF-PHIs as an alternative to ESAs for anemia in NDD-CKDs.
Keywords
Chronic kidney disease
Renal anemia
Non-dialysis dependent
Hypoxia-inducible Factor Prolyl Hydroxylase Inhibitors
Introduction
Chronic kidney disease (CKD) is a progressive disease with various complications.1 Anemia is a common complication of CKD, and depending on the stage of CKD, the coprevalence is estimated to be between 7% and 50%.2 As kidney function declines, the incidence of anemia and its severity also increase, resulting in poor clinical outcomes such as reduced health-related quality of life (HRQoL), increased risk of CKD progression, cardiovascular events, and all-cause mortality. Treatments such as iron supplements, erythropoiesis-stimulating agents (ESAs), and blood transfusions are the current standards of care, though each carries potential risks, side effects, and effectiveness, including an increased risk of cardiovascular events, transfusion-related reactions, thrombosis, and all-cause mortality.3
The presence of anemia in CKD may accelerate progression to dialysis dependence, cardiovascular complications, and premature death.4 As such, treatment of anemia is an important part of CKD management. Iron therapy, ESAs, and rescue blood transfusions are the available options.5 Newer options for anemia in CKD have thus been constantly evaluated.6 Hypoxia-inducible factor prolyl hydroxylase Inhibitors (HIF-PHI) have recently been approved for use in various countries for the condition.7
HIF-PHIs are a new orally administered drug that increases HIF levels and therefore to an increase in endogenous erythropoietin (EPO). Research on HIF-PHI is ongoing and drugs are being approved for the treatment of anemia in adults with CKD in India.8
We aimed to analyze safety and effectiveness of HIF-PHI molecules for treatment of anemia in people with non-dialysis dependent chronic kidney disease (NDD-CKD) to inform the development of a clinical practice guideline in South Asia.9
Our systematic review synthesizes evidence for each HIF-PHIs molecule separately for people with NDD-CKD who have anemia. Our analysis is more nuanced and in alignment with how clinical practice is affected compared to other evidence synthesis pools data from all HIF-PHI molecules together or are on a single HIF-PHI molecule, but with data from dialysis dependent (DD)-CKD and NDD-CKD people with anemia pooled together. It is well known that different HIF-PHI molecules have different safety profiles (the reason they are being developed), making them not interchangeable. Pooling data from all HIF-PHIs together might give a false sense of safety. The profile of DD-CKD and NDD-CKD patients are substantially different with their management being affected differently. A systematic review of HIF-PHIs in DD-CKD patients is presented separately.
Materials and Methods
This review is reported in accordance with the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) 2020 guidelines; the PRISMA checklist is presented in Appendix 1. The protocol was registered a priori in the Open Science Framework (OSF) (https://osf.io/rm7jt).
Criteria for considering studies for this review
We included studies which met the following criteria:
-
Population/participants: Adult patients (≥18 years) of CKD with a diagnosis of anemia not on dialysis. We did not include studies exclusively, including patients with primary anemia, due to systemic causes like bone marrow aplasia or pure red cell aplasia, thalassemia major, sickle cell disease or myelodysplastic syndrome, untreated pernicious anemia, or anemia secondary to other causes such as blood loss due to gastrointestinal (GI) bleeding, cancer, and infectious diseases. If a study involved both adults and children or adolescents, we included only if the disaggregated data on adults was reported in the full text. Anemia and CKD diagnostic criteria used was as defined by the primary authors.
-
Intervention: HIF-PHI administered, including but not limited to Daprodustat, Desidustat, Enarodustat, Molidustat, Roxadustat, Vadadustat. We included studies irrespective of their dosage and frequency of administration.
-
Comparison: We included studies with comparator as standard care for anemia irrespective of whether it contains ESAs, including but not limited to epoetin alpha or darbepoetin alpha administered by any route.
-
Study designs: Randomized controlled trials.
-
Types of outcome measures: We included studies reporting the following outcomes:
Change in hemoglobin levels from baseline
All-cause mortality
Need for iron supplementation
Need for ESA
HRQoL (measured by any validated tool)
Fatigue (measured by any validated scale)
Incidences of Major Adverse Cardiovascular Events (MACE) and MACE plus (as defined by trial authors)
Treatment emergent adverse events (TEAEs)
Progression to end-stage kidney disease
Patient requiring blood transfusion
For the validated tools, all scales operate in the same direction and higher scores indicate greater satisfaction. We captured all time points (above six months), at which the outcomes were measured that were determined by the included studies, explicitly mentioned in the review report. Outcome time points were captured at baseline and up to the maximal time point available. There were few outcomes recorded at multiple time points; thus we assumed the maximal time point available to be equal to the length of follow-up if not specifically mentioned. We assessed outcome measures as per the following: up to 12 months as short term and greater than 12 months as long term.
An inclusive outcome measurement/definition approach was followed to enable capturing of the maximal evidence such that outcomes measured in terms of frequency/proportion or any other modality were included.
Other restrictions
We did not include studies published in non-English languages (where a publicly available translation was not available) and which were available in abstract form only (with no full-length publication available). Authors of studies were not contacted for full texts. We did not restrict by publication date.
Information sources
Electronic database search
A search strategy was developed in PubMed, which was adapted for other electronic databases. The electronic databases searched were PubMed, EMBASE, CINAHL, The Cochrane Central Register of Controlled Trials, Trial registries [clinicaltrials.gov, World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), Clinical Trials Registry - India (CTRI), Sri Lanka Clinical Trials Registry (SLCTR)].
We presented search strategies for all databases, including trial registries, within the full systematic review report to enable transparency and reproducibility. All search strategies are presented in Appendix 2.
Other search methods
The guideline development group members1 were contacted to identify additional studies that potentially meet eligibility criteria. The reference lists of studies that meet eligibility criteria and those retrieved by other modalities of search were manually screened for identifying newer studies.
Data collection and analysis
Selection of studies
At least two review authors independently screened the title and/or abstracts from electronic database search for relevance using the web application Rayyan.10 This was followed by full text articles evaluation against inclusion criteria by at least two review authors. Any discrepancies were resolved by consensus with the other review author.
Data extraction and management
At least two reviewers independently extracted data as per a predesigned data extraction form. Disagreements were resolved by consensus between two authors, with a third author acting as arbiter. Authors of studies were not contacted for additional data and only data as reported in published versions were included.
Assessment of risk of bias in included studies
Risk of bias was assessed by two reviewers independently. The second reviewer used Robot Reviewer11 to facilitate risk of bias assessment, but all assessments were manually checked. A third reviewer was involved for consensus decisions if required. We used Cochrane Risk of Bias 1.0 tool developed by Cochrane.12
Measures of treatment effect
The measures of effect used depended on the type of outcome data.
For dichotomous outcomes (all-cause mortality, need for iron supplementation, need for ESA, incidence of MACE and/or MACE Plus, TEAEs and patients requiring blood transfusion, progression to end-stage kidney disease) odds ratio (OR) with 95% confidence intervals (CI) were used.
For continuous outcomes (change in the hemoglobin level, HRQoL, and fatigue), mean difference (MD) with 95% CI (where included studies report outcomes measured on the same scale) or standardized MD with 95% CI (where included studies report the same outcome measured differently) was used.
Unit of analysis issues
The unit of analysis was the individual participant.
Data synthesis
We summarized results of the included studies narratively and conducted meta-analysis where applicable as per guidance in the Cochrane Handbook for Systematic Reviews of Interventions. Considering expected heterogeneity, we used a random effects approach for meta-analysis. Conducting meta-analysis with a fixed effects model in the presence of even minor heterogeneity may provide overly narrow CIs. We used the Chi2 test and the I2 measure to quantify heterogeneity, but we did not use these to guide the choice of model for meta-analysis. We had planned to do subgroup analysis but could not because of lack of studies.
Dealing with missing data
Investigators for included studies were not contacted to obtain any missing numerical outcome data owing to the time frame in which the systematic review was being conducted. As such, when missing data are encountered, estimations were made as per methods described in the Cochrane Handbook (Chapter 10.12.2). Where this was not possible, we presented the available data along with a note on the issue.
Assessment of heterogeneity
Clinical and methodological heterogeneity was evaluated by generating descriptive statistics for trial, study population, intervention, outcome, setting, and characteristics such as length of follow-up and more across all eligible trials that compared each pair of interventions. This assessment of clinical and methodological heterogeneity was supplemented by information regarding statistical heterogeneity. Heterogeneity of included studies of a particular intervention outcomes pair was assessed by visual inspection of forest plots, the formal homogeneity test, and the evaluation of the proportion of variability due to heterogeneity rather than sampling error.13
Assessment of reporting biases
Funnel plots were used to assess for the potential existence of study bias, if enough studies (at least ten) were available, using standard methods.13
Certainty of evidence from trials
We used the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach to estimate certainty of evidence as per the GRADE handbook.14 We used the GRADE Pro GDT software (https://gradepro.org) to create a Summary of Findings (SoF) table for all primary outcomes. The SoF table presented a maximum of seven outcomes, including adverse events in the SoF table. In the GRADE approach, certainty of evidence was classified as very low, low, moderate, and high by the consensus of the review team (involving at least two authors). Randomized controlled trials (RCTs) were started with high-quality ratings. We reduced or downgraded the certainty of evidence based on the factors listed below, using methods described in the GRADE handbook.
Five factors that can lower confidence in the estimate of an effect, that is, lower the quality of evidencewere study limitations (risk of bias), inconsistency of results, indirectness of evidence, imprecision, publication bias.
Difference between protocol and full review
Patient requiring blood transfusion was not an a priori outcome in the protocol. This was added to capture additional evidence reported in trials which could be useful for decision-making.
Results
We identified 838 studies from database searches, and following removal of 118 duplicates, we screened 720 records based on titles and/or abstracts. We retrieved full texts of 160 studies which were deemed to be potentially eligible for further examination. On full text screening, 12 studies were included in this report.15-26 Figure 1 shows the PRISMA study selection flow chart. The list of excluded studies with reasons for exclusion at the full text level is presented in Appendix 3.
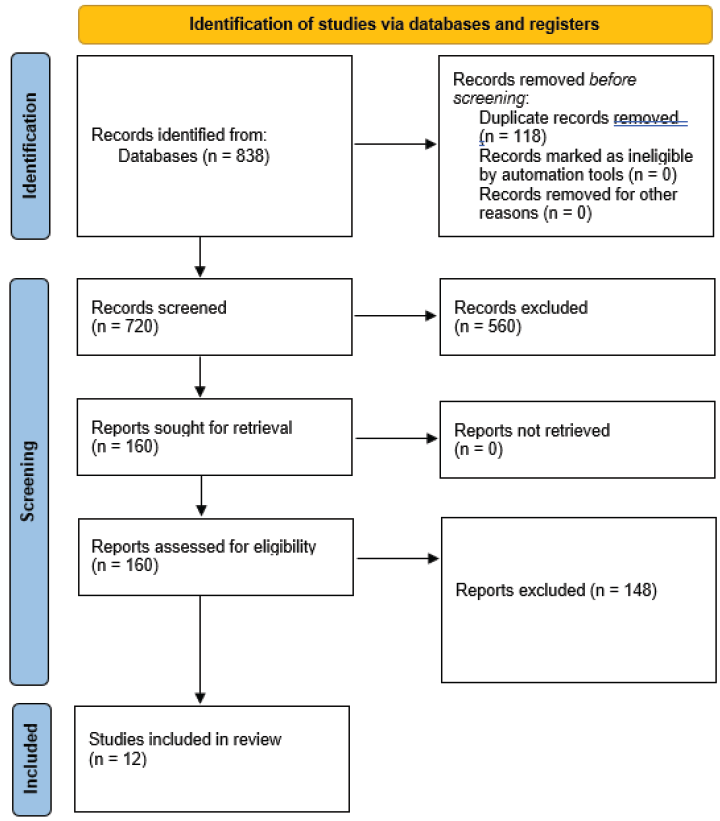
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart showing selection of studies.
Characteristics of included studies
We found 12 studies involving 8611 renal anemia patients assessing efficacy and safety of six HIF-PHI compounds in NDD-CKD patients. The studies included roxadustat (n = 2), daprodustat (n = 3), molidustat (n = 3), vadadustat (n = 2), enarodustat (n = 1), and desidustat (n = 1). We found four trials conducted on ESA-naïve patients, six on both ESA-conditioned and naïve patients, and three on ESA-conditioned patients. The treatment duration ranged from 24 weeks to 2.1 years. All characteristics of the studies are summarized in Appendix 4.
Quality assessment of included studies
The risk of bias summary for the 26 included RCTs is presented in Figure 2.
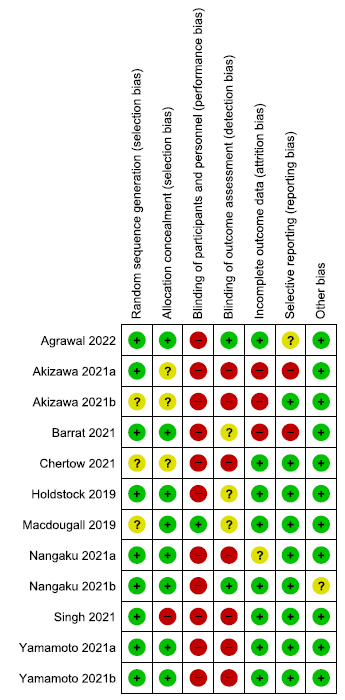
- Risk of bias summary for included randomized controlled trials. Low risk of bias is signified by the green circles with ‘+’ symbols, Unclear risk of biase is signified by the yellow circles with ‘?’ symbol, and High risk of bias is signified by the red circles with ‘-’ symbol.
Effect of different HIF-PHIs on people with NDD-CKD
All GRADE evaluations are presented in Appendix 5.
Effect of HIF-PHI on the change in hemoglobin levels from baseline
We found ten studies reporting the effect of HIF-PHIs on the change in hemoglobin from baseline as compared to ESAs.
Effect of desidustat versus darbepoetin alpha on change in hemoglobin levels from baseline up to 24 weeks
One study reported change in hemoglobin levels from baseline up to 24 weeks in desidustat as compared to darbepoetin alpha. Desidustat reported little or no difference in the hemoglobin levels from baseline up to 24 weeks as compared to darbepoetin alpha (MD: 0.09 g/dL [CI 95% -0.15–0.33]; p = 0.46; 529 participants; low certainty evidence).15 The forest plot is shown in Figure 3.
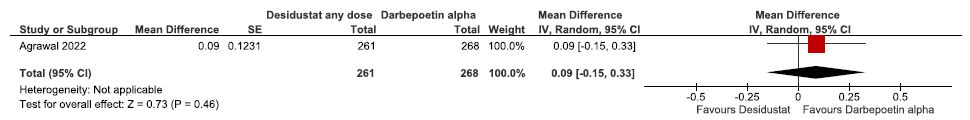
- Forest plot for desidustat versus darbepoetin alpha on the change in hemoglobin levels from baseline up to 24 weeks. CI: Confidence interval, SE: Standard error, IV: inverse variance
Effect of daprodustat versus rhEPO (epoetins or their biosimilars or darbepoetin) on the change in hemoglobin levels from baseline up to 52 weeks
Two studies reported the change in hemoglobin levels from baseline up to 52 weeks in daprodustat as compared to rhEPO. The pooled results reported daprodustat had little or no difference in the change in hemoglobin levels from baseline up to 52 weeks as compared to rhEPO (MD: 0.08 g/dL [CI 95% 0.08–0.08]; p < 0.00001; two studies; 4089 participants; low certainty evidence).16,17 The forest plot is shown in Figure 4.
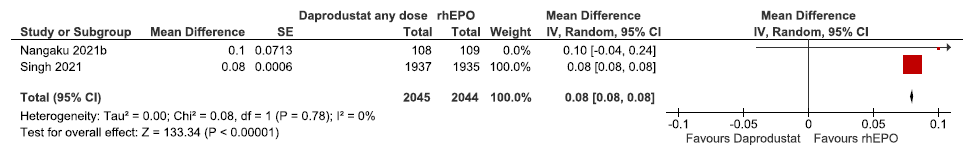
- Forest plot for daprodustat versus rhEPO on the change in hemoglobin levels from baseline up to 52 weeks. CI: Confidence interval, SE: Standard error, IV: inverse variance, rhEPO: Epoetins or their biosimilars or darbepoetin, df: degrees of freedom
Effect of enarodustat versus darbepoetin alpha on the change in hemoglobin levels from baseline up to 24 weeks
One study reported the change in hemoglobin levels from baseline up to 24 weeks in enarodustat as compared to darbepoetin alpha. Enarodustat had little or no difference in the change in hemoglobin levels from baseline up to 24 weeks as compared to darbepoetin alpha (MD: 0.09 g/dL [CI 95% -0.08–0.26); p = 0.29; 193 participants; very low certainty evidence).18 The forest plot is shown in Figure 5.
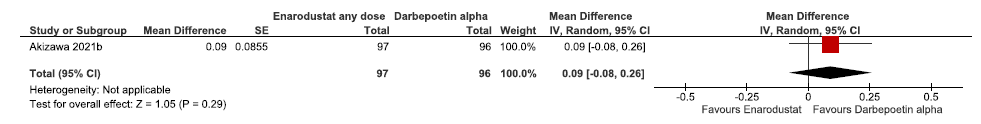
- Forest plot for enarodustat versus darbepoetin alpha on the change in hemoglobin levels from baseline up to 24 weeks. CI: Confidence interval, SE: Standard error, IV: inverse variance
Effect of molidustat versus darbepoetin alpha on the change in hemoglobin levels from baseline up to 36 weeks
Three studies reported the change in hemoglobin levels from baseline up to 36 weeks in molidustat as compared to darbepoetin alpha. The pooled results reported molidustat reduced the hemoglobin levels from baseline up to 36 weeks as compared to darbepoetin alpha (MD: -0.11 g/dL [CI 95% -0.52–0.30]; p = 0.60; three studies; 434 participants; very low certainty evidence).19–21 The forest plot is shown in Figure 6.
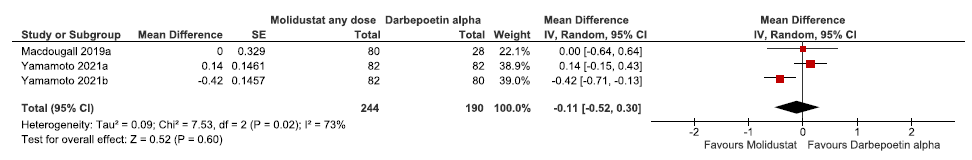
- Forest plot for molidustat versus darbepoetin alpha on the change in hemoglobin levels from baseline up to 36 weeks. CI: Confidence interval, SE: Standard error, IV: inverse variance, df: degrees of freedom
Effect of roxadustat versus darbepoetin alpha on the change in hemoglobin levels from baseline up to 24 weeks
One study reported the change in hemoglobin levels from baseline up to 24 weeks in roxadustat as compared to darbepoetin alpha. Roxadustat reduced the hemoglobin levels from baseline up to 24 weeks as compared to darbepoetin alpha (MD: -0.12 g/dL [CI 95% -0.30–0.06]; p = 0.19; 262 participants; very low certainty evidence).22 The forest plot is shown in Figure 7.
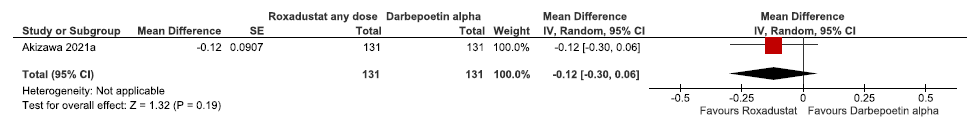
- Forest plot for roxadustat versus darbepoetin alpha on the change in hemoglobin levels from baseline up to 24 weeks. CI: Confidence interval, SE: Standard error, IV: inverse variance
Effect of vadadustat versus darbepoetin alpha on the change in hemoglobin levels from baseline up to 52 weeks
Two studies (three trials) reported the change in hemoglobin levels from baseline up to 52 weeks in vadadustat as compared to darbepoetin alpha. The pooled results reported vadadustat had little or no difference on the hemoglobin levels from baseline up to 52 weeks as compared to darbepoetin alpha (MD: 0.00 g/dL [CI 95% -0.04–0.05]; p = 0.87; 2 studies; 3780 participants; very low certainty evidence).23,24 The forest plot is shown in Figure 8.
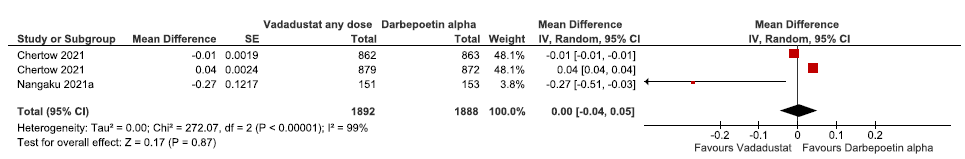
- Forest plot for vadadustat versus darbepoetin alpha on the change in hemoglobin levels from baseline up to 52 weeks. CI: Confidence interval, SE: Standard error, IV: inverse variance, df: degrees of freedom
Effect of HIF-PHI on all-cause mortality
We found 11 studies reporting the effect of HIF-PHIs on all-cause mortality as compared to ESAs.
Effect of desidustat versus darbepoetin alpha on all-cause mortality up to 26 weeks
One study reported all-cause mortality up to 26 weeks in desidustat as compared to darbepoetin alpha. Desidustat had no difference on all-cause mortality up to 26 weeks as compared to darbepoetin alpha (Odds ratio (OR): 1 [CI 95% 0.32–3.14]; p = 1.00; 588 participants; low certainty evidence).15 The forest plot is shown in Figure 9.
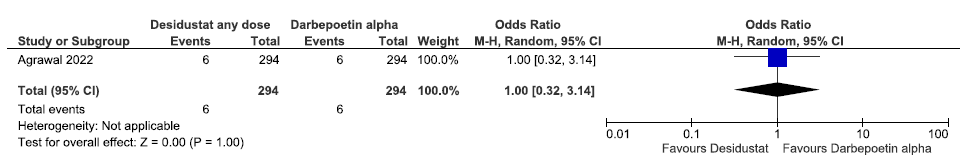
- Forest plot for desidustat versus darbepoetin alpha on all-cause mortality up to 26 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of daprodustat versus rhEPO (epoetins or their biosimilars or darbepoetin) on all-cause mortality up to 52 weeks
One study reported all-cause mortality up to 52 weeks in daprodustat as compared to rhEPO. Daprodustat increased all-cause mortality up to 52 weeks as compared to rhEPO (OR: 1.90 [CI 95% 0.21–17.31]; p = 0.57; 250 participants; very low certainty evidence).25 The forest plot is shown in Figure 10.
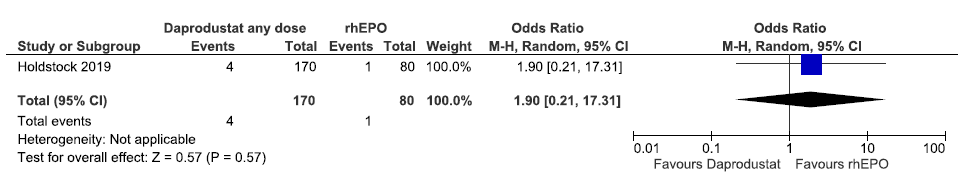
- Forest plot for daprodustat versus rhEPO on all-cause mortality up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, rhEPO: Epoetins or their biosimilars or darbepoetin
Effect of daprodustat versus rhEPO (epoetins or their biosimilars or darbepoetin) on all-cause mortality up to 60 weeks
One study reported all-cause mortality up to 60 weeks in daprodustat as compared to rhEPO. Daprodustat had little or no difference on all-cause mortality up to 60 weeks as compared to rhEPO (OR: 1.01 [CI 95% 0.85–1.20]; p = 0.90; 3872 participants; very low certainty evidence).17 The forest plot is shown in Figure 11.
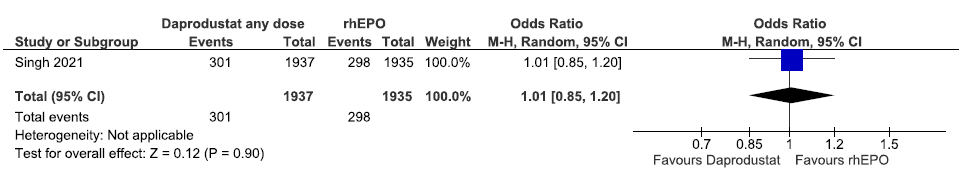
- Forest plot for daprodustat versus rhEPO on all-cause mortality up to 60 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, rhEPO: Epoetins or their biosimilars or darbepoetin
Effect of enarodustat versus darbepoetin alpha on all-cause mortality up to 26 weeks
One study reported all-cause mortality up to 26 weeks in enarodustat as compared to darbepoetin alpha. Enarodustat decreases all-cause mortality up to 26 weeks as compared to darbepoetin alpha [OR: 0.34; (CI 95% 0.01–8.35); p = 0.51; 216 participants; very low certainty evidence].18 The forest plot is shown in Figure 12.
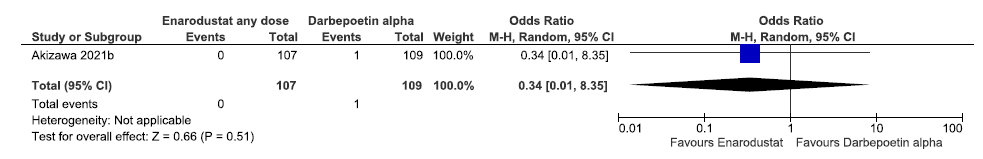
- Forest plot for enarodustat versus darbepoetin alpha on the change in all-cause mortality up to 26 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of molidustat versus darbepoetin alpha on all-cause mortality up to 52 weeks
Three studies reported all-cause mortality up to 52 weeks in molidustat as compared to darbepoetin alpha. The pooled results reported molidustat increased the all-cause mortality up to 52 weeks as compared to darbepoetin alpha [OR: 1.78 (CI 95% 0.38–8.28); p = 0.46; 3 studies; 449 participants; very low certainty evidence].19–21 The forest plot is shown in Figure 13.
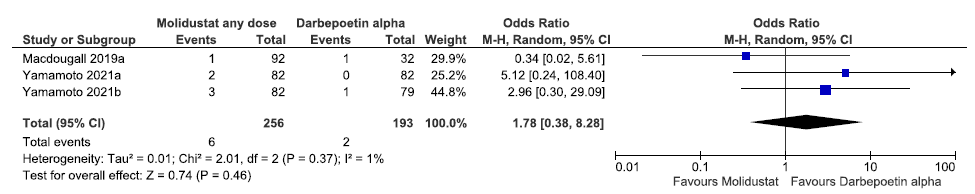
- Forest plot for molidustat versus darbepoetin alpha on all-cause mortality up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, df: degrees of freedom, ESA: Eythropoiesis-stimulating agents
Effect of roxadustat versus darbepoetin alpha on all-cause mortality up to 52 weeks
One study reported all-cause mortality up to 52 weeks in roxadustat as compared to darbepoetin alpha. Roxadustat decreased all-cause mortality up to 52 weeks as compared to darbepoetin alpha [OR: 0.33 (CI 95% 0.01–8.19); p = 0.50; 262 participants; very low certainty evidence].22 The forest plot is shown in Figure 14.
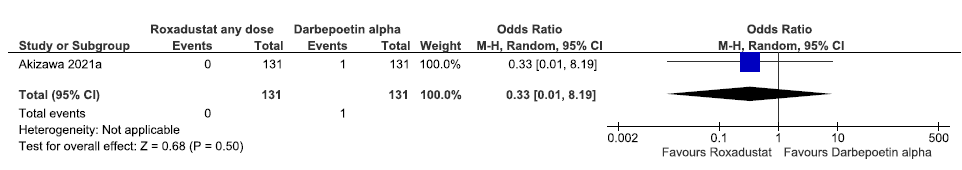
- Forest plot for roxadustat versus darbepoetin alpha on all-cause mortality up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of roxadustat versus darbepoetin alpha on all-cause mortality up to 108–209 weeks
One study reported all-cause mortality up to 108–209 weeks in roxadustat as compared to darbepoetin alpha. The pooled results reported roxadustat increased all-cause mortality up to 108–209 weeks as compared to darbepoetin alpha [OR: 0.87 (CI 95% 0.51–1.47); p = 0.59; 616 participants; very low certainty evidence].26 The forest plot is shown in Figure 15.
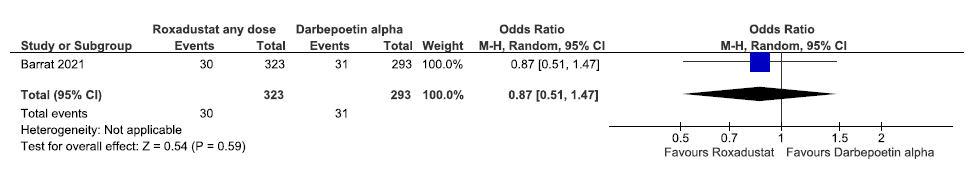
- Forest plot for roxadustat versus darbepoetin alpha on all-cause mortality up to 108 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of vadadustat versus darbepoetin alpha on all-cause mortality up to 52 weeks
One study reported all-cause mortality up to 52 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat decreased all-cause mortality up to 52 weeks as compared to darbepoetin alpha [OR: 0.34 (CI 95% 0.01–8.30); p = 0.50; 304 participants; very low certainty evidence].24 The forest plot is shown in Figure 16.
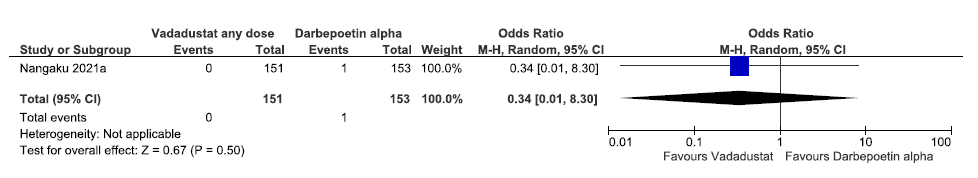
- Forest plot for vadadustat versus darbepoetin alpha on all-cause mortality up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of vadadustat versus darbepoetin alpha on all-cause mortality up to 57 weeks
One study reported all-cause mortality up to 57 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat had little or no difference on all-cause mortality up to 57 weeks as compared to darbepoetin alpha [OR: 1.01 (95% CI 0.85–1.20); p = 0.93; 3521 participants; very low certainty evidence].23 The forest plot is shown in Figure 17.
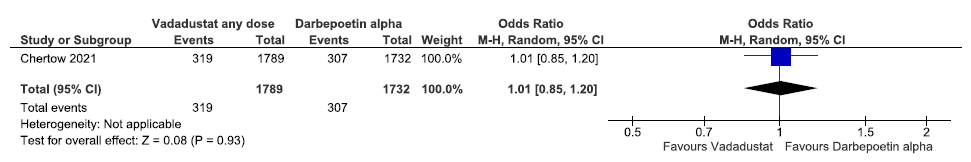
- Forest plot for vadadustat versus darbepoetin alpha on all-cause mortality up to 57 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of HIF-PHI on the need for oral/intravenous iron supplementation
We found four studies reporting the effect of HIF-PHIs on the need for iron supplementation as compared to ESAs.
Effect of molidustat versus darbepoetin alpha on need for oral iron supplementation up to 52 weeks
Two studies reported the need for oral iron supplementation up to 52 weeks in molidustat as compared to darbepoetin alpha. The pooled results reported that molidustat increased the need for oral iron supplementation up to 52 weeks as compared to darbepoetin alpha [OR: 1.71 (95% CI 1.10–2.66); p = 0.02; two studies; 325 participants; very low certainty evidence].20,21 The forest plot is shown in Figure 18.
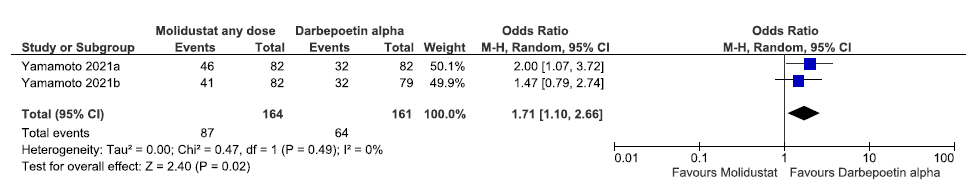
- Forest plot for molidustat versus darbepoetin alpha on the need for oral iron supplementation up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, df: degrees of freedom
Effect of molidustat versus darbepoetin alpha on the need for IV iron supplementation up to 52 weeks
Two studies reported the need for IV iron supplementation up to 52 weeks in molidustat as compared to darbepoetin alpha. The pooled results reported molidustat has little or no difference on the need for IV iron supplementation up to 52 weeks as compared to darbepoetin alpha [OR: 0.97 (95% CI 0.31–3.09); p = 0.96; 325 participants; very low certainty evidence].20,21 The forest plot is shown in Figure 19.
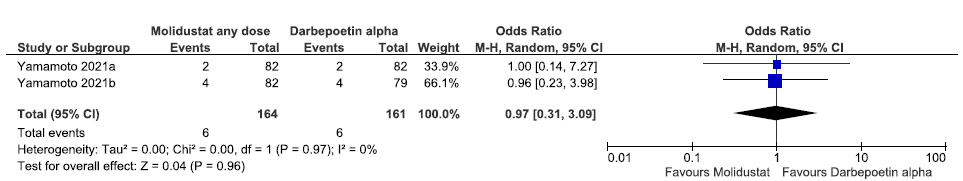
- Forest plot for molidustat versus darbepoetin alpha on the need for IV iron supplementation up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, df: degrees of freedom, IV:Intra venous
Effect of roxadustat versus darbepoetin alpha on the need for bivalent oral iron supplementation up to 36 weeks
One study reported the need for bivalent oral iron supplementation up to 36 weeks in roxadustat as compared to darbepoetin alpha. Roxadustat decreased the need for bivalent oral iron supplementation up to 36 weeks as compared to darbepoetin alpha [OR: 0.78 (95% CI 0.57–1.07); p = 0.13; 616 participants; very low certainty evidence].26 The forest plot is shown in Figure 20.
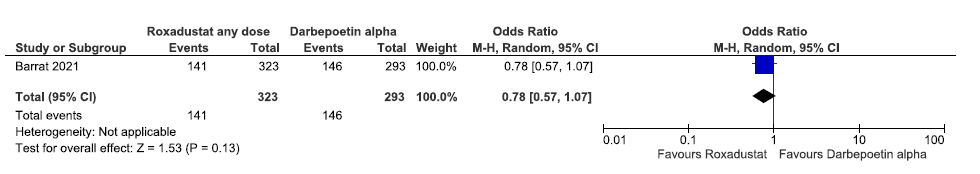
- Forest plot for roxadustat versus darbepoetin alpha on the need for bivalent oral iron supplementation up to 36 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of roxadustat versus darbepoetin alpha on the need for trivalent oral iron supplementation up to 36 weeks
One study reported the need for trivalent oral iron supplementation up to 36 weeks in roxadustat as compared to darbepoetin alpha. Roxadustat decreased the need for trivalent oral iron supplementation up to 36 weeks as compared to darbepoetin alpha [OR: 0.67 (95% CI 0.49–0.93); p = 0.02; 616 participants; very low certainty evidence].26 The forest plot is shown in Figure 21.
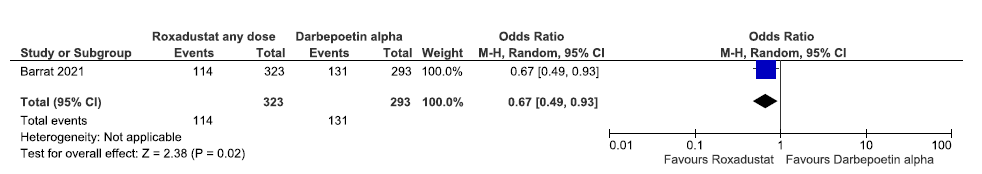
- Forest plot for roxadustat versus darbepoetin alpha on the need for trivalent oral iron supplementation up to 36 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of roxadustat versus darbepoetin alpha on the need for IV iron supplementation up to 36 weeks
One study reported the need for IV iron supplementation up to 36 weeks in roxadustat as compared to darbepoetin alpha. Roxadustat decreased the need for IV iron supplementation up to 36 weeks as compared to darbepoetin alpha [OR: 0.46 (95% CI 0.26–0.81); p = 0.007; 616 participants; very low certainty evidence].26 The forest plot is shown in Figure 22.
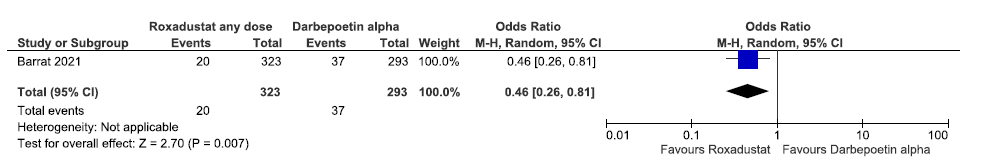
- Forest plot for roxadustat versus darbepoetin alpha on the need for IV iron supplementation up to 36 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, IV: Intra venous
Effect of vadadustat versus darbepoetin alpha on the need for oral iron supplementation up to 52 weeks
One study reported the need for oral iron supplementation up to 52 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat increased the need for oral iron supplementation up to 52 weeks as compared to darbepoetin alpha [OR: 1.26 (95% CI 0.78–2.05); p = 0.35; 302 participants; very low certainty evidence].24 The forest plot is shown in Figure 23.
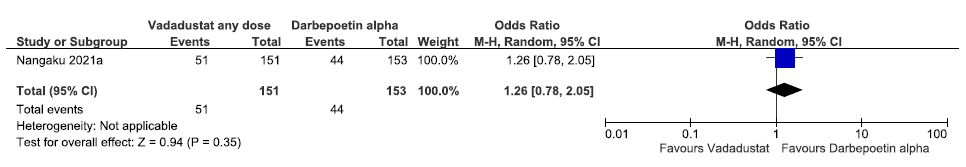
- Forest plot for vadadustat versus darbepoetin alpha on the need for oral iron supplementation up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of HIF-PHI on the need for ESA
We found four studies reporting the effect of HIF-PHIs on the need for ESA as compared to ESAs.
Effect of desidustat versus darbepoetin alpha on need for ESA up to 24 weeks
One study reported need for ESA up to 24 weeks in desidustat as compared to darbepoetin alpha. There were too few patients who experienced the need for ESA up to 24 weeks, to determine whether desidustat made a difference as compared to darbepoetin alpha (OR: Not estimable; 588 participants; low certainty evidence).15 The forest plot is shown in Figure 24.
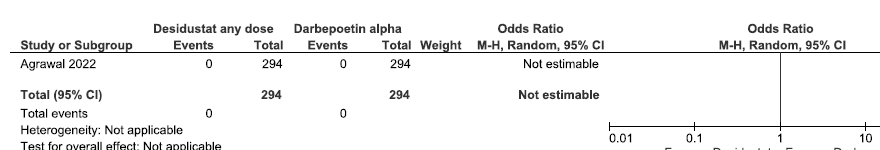
- Forest plot for desidustat versus darbepoetin alpha on the need for ESA up to 24 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, ESA: Erythropoiesis-stimulating agents
Effect of molidustat versus darbepoetin alpha on the need for ESA up to 36 weeks
Three studies reported the need for ESA up to 36 weeks in molidustat as compared to darbepoetin alpha. The pooled results reported molidustat decreased the need for ESA up to 36 weeks as compared to darbepoetin alpha [OR: 0.39 (95% CI 0.11–1.42); p = 0.15; three studies; 449 participants; very low certainty evidence].19–21 The forest plot is shown in Figure 25.
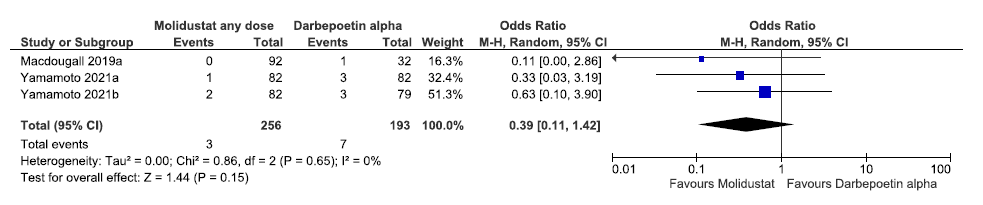
- Forest plot for molidustat versus darbepoetin alpha on the need for ESA up to 36 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, df: degrees of freedom, ESA: Eythropoiesis-stimulating agents
Effect of HIF-PHI on HRQoL
We found one study reporting effect of HIF-PHIs on HRQoL as compared to ESAs.
Effect of desidustat versus darbepoetin alpha on the QoL assessed by SF-36 up to 24 weeks
One study reported QoL assessed by SF-36 up to 24 weeks in desidustat as compared to darbepoetin alpha. The study reported desidustat has no difference on the QoL assessed by SF-36 up to 24 weeks as compared to darbepoetin alpha [MD: 0.00 (95% CI -98.20–98.20); p = 1.0; 480 participants; low certainty evidence].15 The forest plot is shown in Figure 26.
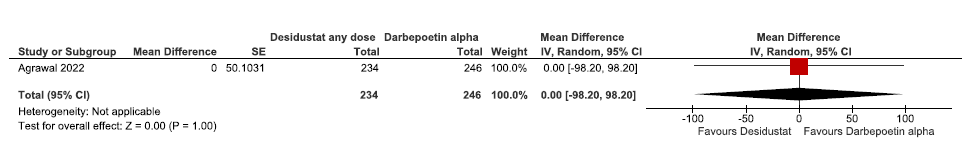
- Forest plot for desidustat versus darbepoetin alpha on the QoL assessed by SF-36 up to 24 weeks. CI: Confidence interval, SE: Standard error, QoL: Quality of life, SF-36: 36-Item Short Form
Effect of HIF-PHI on fatigue
We did not find any study reporting effect of HIF-PHIs on fatigue as compared to ESAs.
Effect of HIF-PHI on incidences of MACE and MACE plus
We found six studies reporting effect of HIF-PHIs on incidences of MACE and MACE plus as compared to ESAs.
Effect of daprodustat versus rhEPO (epoetins or their biosimilars or darbepoetin) on incidences of MACE up to 60 weeks
One study reported incidences of MACE up to 60 weeks in daprodustat as compared to rhEPO. The results reported daprodustat increased incidences of MACE up to 60 weeks as compared to rhEPO [OR: 1.07 (95% CI 0.92–1.24); p = 0.39; 3872 participants; very low certainty evidence].17 The forest plot is shown in Figure 27.
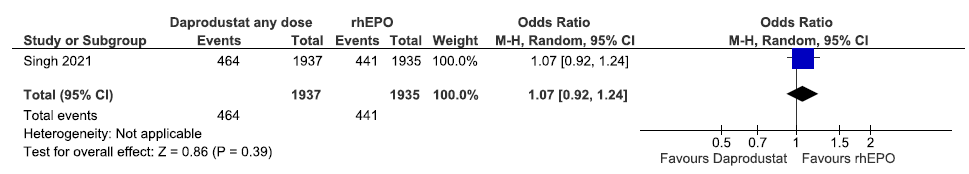
- Forest plot for daprodustat versus rhEPO on incidences of MACE up to 60 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, MACE: Major adverse cardiovascular events, rhEPO: Epoetins or their biosimilars or darbepoetin
Effect of daprodustat versus rhEPO (epoetins or their biosimilars or darbepoetin) on incidences of MACE plus up to 32 weeks
One study reported incidences of MACE plus up to 32 weeks in daprodustat as compared to rhEPO. The results reported daprodustat decreased incidences of MACE plus up to 32 weeks as compared to rhEPO [OR: 0.82 (95% CI 0.23–2.870); p = 0.75; 250 participants; very low certainty evidence].25 The forest plot is shown in Figure 28.
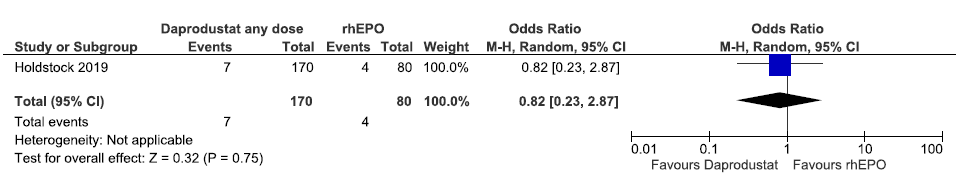
- Forest plot for daprodustat versus rhEPO on incidences of MACE plus up to 32 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, MACE: Major adverse cardiovascular events, rhEPO: Epoetins or their biosimilars or darbepoetin
Effect of molidustat versus darbepoetin alpha on incidences of MACE up to 52 weeks
Two studies reported incidences of MACE up to 52 weeks in molidustat as compared to darbepoetin alpha. Molidustat increased the incidences of MACE up to 52 weeks as compared to darbepoetin alpha [OR: 5.43 (95% CI 0.90–32.61); p = 0.06; two studies; 325 participants; very low certainty evidence].20,21 The forest plot is shown in Figure 29.
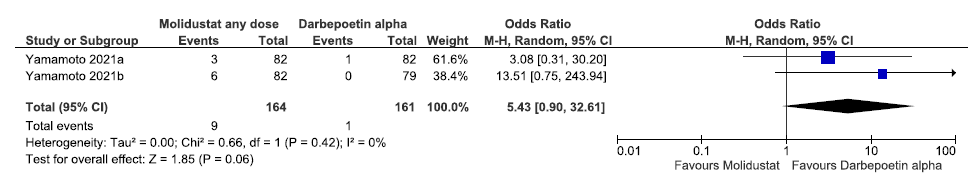
- Forest plot for molidustat versus darbepoetin alpha on incidences of MACE up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, MACE: Major adverse cardiovascular events, df: degrees of freedom
Effect of roxadustat versus darbepoetin alpha on incidences of MACE up to 108 weeks
One study reported incidences of MACE up to 108 weeks in roxadustat as compared to darbepoetin alpha. Roxadustat decreased incidences of MACE up to 108 weeks as compared to darbepoetin alpha (OR:0.82 [CI 95% 0.51–1.31]; 616 participants; very low certainty evidence).26 The forest plot is shown in Figure 30.
- Forest plot for roxadustat versus darbepoetin alpha on incidences of MACE up to 108 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, MACE: Major adverse cardiovascular events
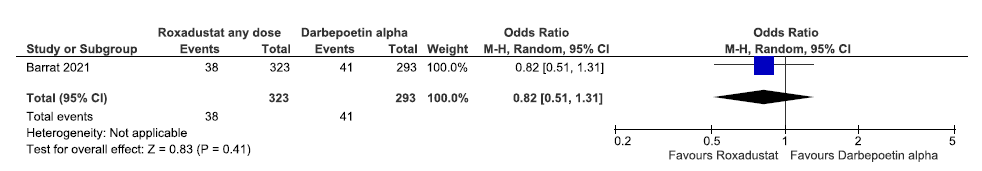
Effect of roxadustat versus darbepoetin alpha on incidences of MACE plus up to 108 weeks
One study reported incidences of MACE plus up to 108 weeks in roxadustat as compared to darbepoetin alpha. Roxadustat decreased incidences of MACE plus up to 108 weeks as compared to darbepoetin alpha [OR:0.91 (CI 95% 0.60–1.38); 616 participants; very low certainty evidence].26 The forest plot is shown in Figure 31.
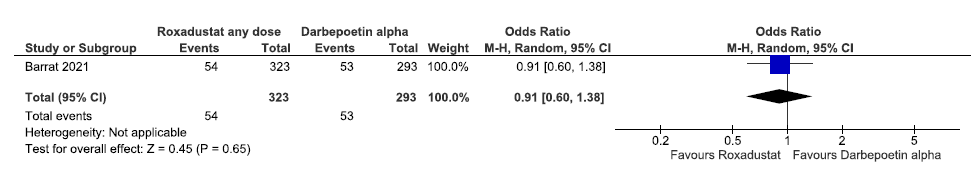
- Forest plot for roxadustat versus darbepoetin alpha on incidences of MACE plus up to 108 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, MACE: Major adverse cardiovascular events
Effect of vadadustat versus darbepoetin alpha on incidences of MACE up to 57 weeks
One study reported incidences of MACE up to 57 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat increased incidences of MACE up to 57 weeks as compared to darbepoetin alpha [OR: 1.10 (95% CI 0.93–1.29); p = 0.27; 3521 participants; very low certainty evidence].23 The forest plot is shown in Figure 32.
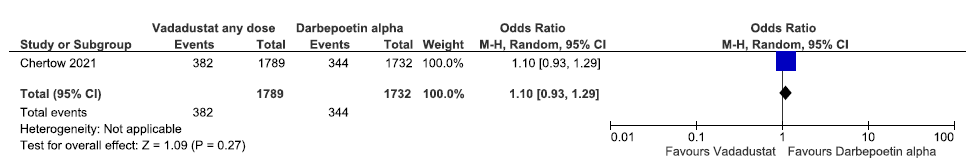
- Forest plot for vadadustat versus darbepoetin alpha on incidences of MACE up to 57 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, MACE: Major adverse cardiovascular events
Effect of vadadustat versus darbepoetin alpha on incidences of MACE plus up to 57 weeks
One study reported incidences of MACE plus up to 57 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat increased incidences of MACE plus up to 57 weeks as compared to darbepoetin alpha [OR: 1.04 (95% CI 0.89–1.21); p = 0.62; 3521 participants; very low certainty evidence].23 The forest plot is shown in Figure 33.
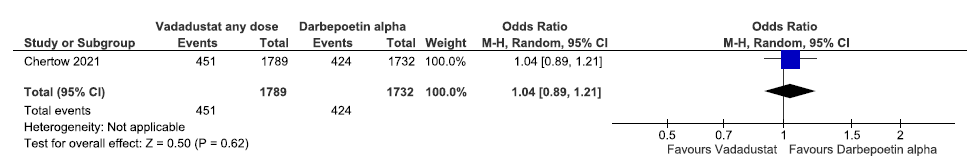
- Forest plot for vadadustat versus darbepoetin alpha on incidences of MACE plus up to 57 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, MACE: Major adverse cardiovascular events
Effect of HIF-PHI on TEAEs
We found 12 studies reporting the effect of HIF-PHIs on TEAEs as compared to ESAs.
Effect of desidustat versus darbepoetin alpha on any adverse events up to 26 weeks
One study reported any adverse events up to 26 weeks in desidustat as compared to darbepoetin alpha. The study reported desidustat decreased any adverse events up to 26 weeks as compared to darbepoetin alpha [OR: 0.91 (95% CI 0.66–1.26); p = 0.56; 588 participants; low certainty evidence].15 The forest plot is shown in Figure 34.
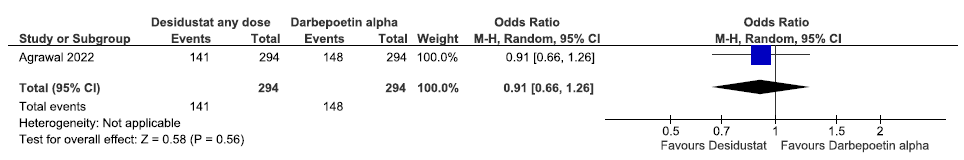
- Forest plot for desidustat versus darbepoetin alpha on any adverse events up to 26 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of daprodustat versus rhEPO (epoetins or their biosimilars or darbepoetin) on adverse events up to 52 weeks
Three studies reported adverse events up to 52 weeks in daprodustat as compared to rhEPO. The pooled results reported daprodustat increased adverse events up to 52 weeks as compared to rhEPO [OR: 1.18 (95% CI 1.02–1.37); p = 0.02; three studies; 4419 participants; low certainty evidence].17,25 The forest plot is shown in Figure 35.
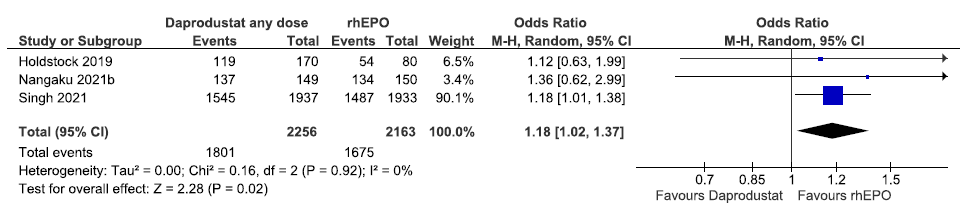
- Forest plot for daprodustat versus rhEPO on adverse events up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, df: degrees of freedom, rhEPO: Epoetins or their biosimilars or darbepoetin
Effect of enarodustat versus darbepoetin alpha on adverse events up to 26 weeks
One study reported adverse events up to 26 weeks in enarodustat as compared to darbepoetin alpha. Enarodustat decreased adverse events up to 26 weeks as compared to darbepoetin alpha [OR: 0.40 (95% CI 0.21–0.75); p = 0.005; 216 participants; very low certainty evidence].18 The forest plot is shown in Figure 36.
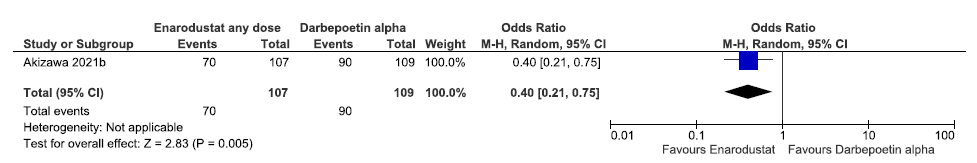
- Forest plot for enarodustat versus darbepoetin alpha on adverse events up to 26 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of molidustat versus darbepoetin alpha on TEAEs up to 52 weeks.
Three studies reported TEAEs up to 52 weeks in molidustat as compared to darbepoetin alpha. The pooled results reported molidustat increased TEAEs up to 52 weeks as compared to darbepoetin alpha [OR: 1.18 (95% CI 0.52–2.67); p = 0.69; three studies; 449 participants; very low certainty evidence].19–21 The forest plot is shown in Figure 37.
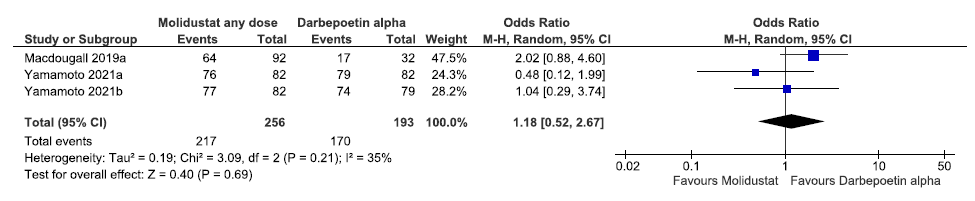
- Forest plot for molidustat versus darbepoetin alpha on TEAEs up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, df: degrees of freedom, TEAE: Treatment emergent adverse events
Effect of roxadustat versus darbepoetin alpha on TEAEs up to 52 weeks
One study reported TEAEs up to 52 weeks in roxadustat as compared to darbepoetin alpha. Roxadustat increased TEAEs up to 52 weeks as compared to darbepoetin alpha [OR: 1.56 (95% CI 0.89–2.73); p = 0.12; 262 participants; very low certainty evidence].22 The forest plot is shown in Figure 38.
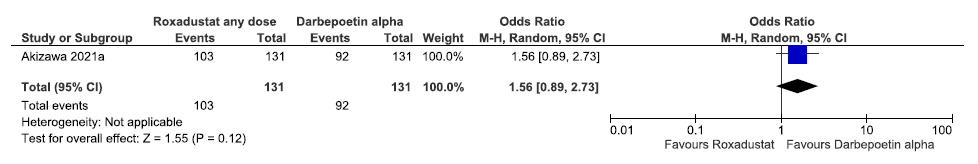
- Forest plot for roxadustat versus darbepoetin alpha on TEAEs up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, TEAE: Treatment emergent adverse events
Effect of roxadustat versus darbepoetin alpha on TEAEs up to 108 weeks
One study reported TEAEs up to 108 weeks in roxadustat as compared to darbepoetin alpha. Roxadustat decreased TEAEs up to 108 weeks as compared to darbepoetin alpha [OR: 0.89 (95% CI 0.50–1.60); p = 0.70; 616 participants; very low certainty evidence].26 The forest plot is shown in Figure 39.
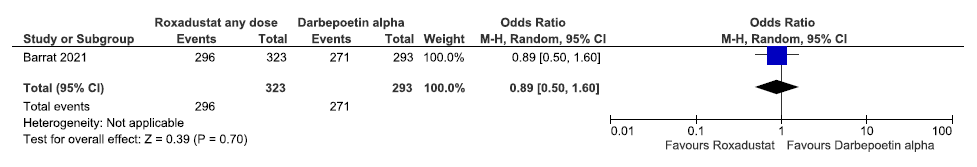
- Forest plot for roxadustat versus darbepoetin alpha on TEAEs up to 108 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, TEAE: Treatment emergent adverse events
Effect of vadadustat versus darbepoetin alpha on adverse events up to 52 weeks
One study reported adverse events up to 52 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat decreased adverse event up to 52 weeks as compared to darbepoetin alpha [OR: 0.77 (95% CI 0.35–1.710; p = 0.52; 304 participants; very low certainty evidence].24 The forest plot is shown in Figure 40.
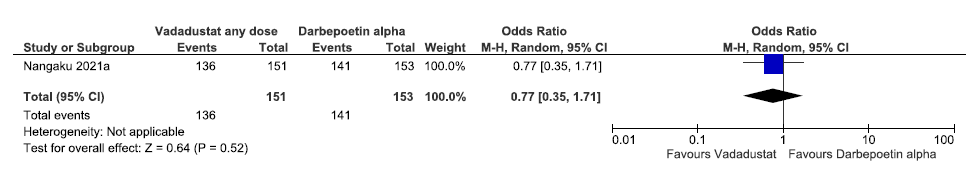
- Forest plot for vadadustat versus darbepoetin alpha on adverse events up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of vadadustat versus darbepoetin alpha on adverse events up to 57 weeks
One study reported adverse events up to 57 weeks in vadadustat as compared to darbepoetin alpha. Vadadustat decreased adverse events up to 57 weeks as compared to darbepoetin alpha [OR: 0.91 (95% CI 0.66–1.27); p = 0.59; 1748 participants; very low certainty evidence].23 The forest plot is shown in Figure 41.
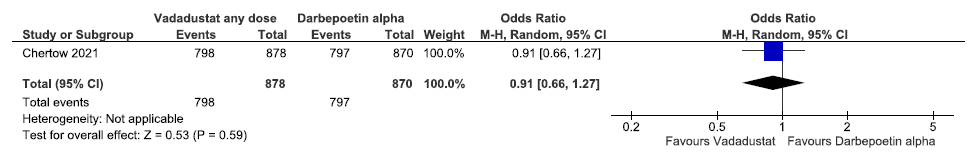
- Forest plot for vadadustat versus darbepoetin alpha on adverse events up to 57 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of HIF-PHI on requirement of blood transfusion
We found five studies reporting the effect of HIF-PHIs on patients requiring blood transfusion as compared to ESAs.
Effect of daprodustat versus rhEPO (epoetins or their biosimilars or darbepoetin) on requirement of blood transfusion up to 52 weeks.
One study reported patients requiring blood transfusion up to 52 weeks in daprodustat as compared to rhEPO. Daprodustat decreased patients requiring blood transfusion up to 52 weeks as compared to rhEPO (OR: 0.94 [95% CI 0.78–1.13]; p = 0.52; 3870 participants; very low certainty evidence).17 The forest plot is shown in Figure 42.
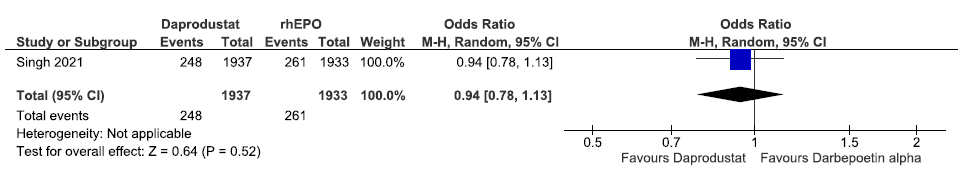
- Forest plot for daprodustat versus rhEPO on patients requiring blood transfusion up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, rhEPO: Epoetins or their biosimilars or darbepoetin
Effect of molidustat versus darbepoetin alpha on requirement of blood transfusion up to 16–52 weeks
Three studies reported patients requiring blood transfusion up to 16–52 weeks in molidustat as compared to darbepoetin alpha. The pooled results reported molidustat decreased patients requiring blood transfusion up to 16–52 weeks as compared to darbepoetin alpha [OR:0.69 (95% CI 0.14–3.47); p = 0.61; three studies; 449 participants; very low certainty evidence].19–21 The forest plot is shown in Figure 43.
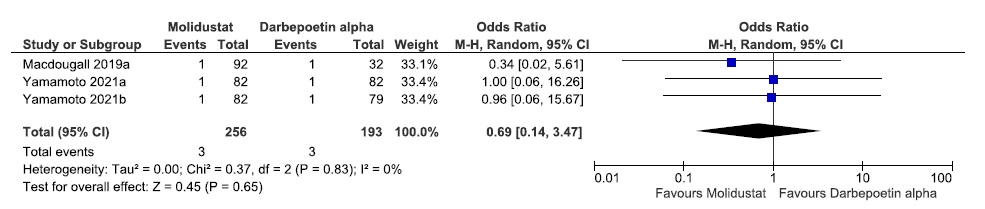
- Forest plot for molidustat versus darbepoetin alpha patients requiring blood transfusion up to 16–52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, df: degrees of freedom
Effect of roxadustat versus darbepoetin alpha on requirement of blood transfusion up to 108 weeks
One study reported patients requiring blood transfusion up to 108 weeks in roxadustat as compared to darbepoetin alpha. Roxadustat increased patients requiring blood transfusion up to 108 weeks as compared to darbepoetin alpha [OR: 1.26 (95% CI 0.75–2.110); p = 0.38; 614 participants; very low certainty evidence].26 The forest plot is shown in Figure 44.
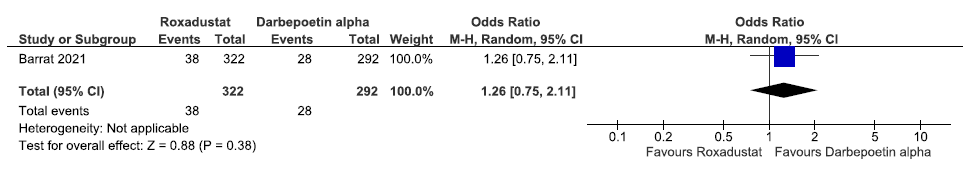
- Forest plot for roxadustat versus darbepoetin alpha on patients requiring blood transfusion up to 108 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method
Effect of HIF-PHI on the progression to end-stage kidney disease.
We found three studies reporting the effect of HIF-PHIs on the progression to end-stage kidney disease as compared to ESAs.
Effect of daprodustat versus rhEPO (epoetins or their biosimilars or darbepoetin) on the progression to end-stage kidney disease up to 60 weeks
One study reported the progression to end-stage kidney disease up to 60 weeks in daprodustat as compared to rhEPO. Daprodustat had no difference in the progression to end-stage kidney disease up to 60 weeks as compared to rhEPO [OR: 0.99 (95% CI 0.83–1.18); p = 0.88; 2485 participants; very low certainty evidence].17 The forest plot is shown in Figure 45.
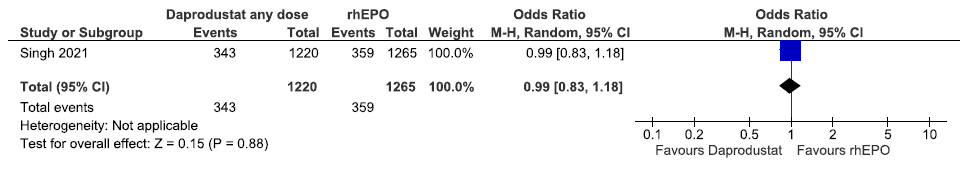
- Forest plot for daprodustat versus rhEPO on the progression to end-stage kidney disease up to 60 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, rhEPO: Epoetins or their biosimilars or darbepoetin
Effect of molidustat versus darbepoetin alpha on the progression to end-stage kidney disease up to 52 weeks
Two studies reported the progression to end-stage kidney disease up to 52 weeks in molidustat as compared to darbepoetin alpha. The pooled results reported molidustat increased the progression to end-stage kidney disease up to 52 weeks as compared to darbepoetin alpha [OR: 1.97 (95% CI 1.04–3.73); p = 0.04; two studies; 325 participants; very low certainty evidence].20,21 The forest plot is shown in Figure 46.
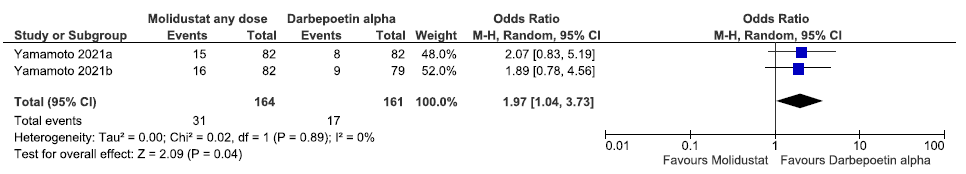
- Forest plot for molidustat versus darbepoetin alpha on the progression to end-stage kidney disease up to 52 weeks. CI: Confidence interval, M-H: Mantel-Haenszel method, df: degrees of freedom
Discussion
We identified 12 randomized trials evaluating the efficacy and safety of HIF-PHI in NDD-CKD patients with anemia. Three studies each were conducted in roxadustat, daprodustat, and molidustat, two in vadadustat, and one each in enarodustat and desidustat. The review highlighted a conspicuous lack of high certainty evidence. Desidustat and daprodustat reported no difference in the hemoglobin levels from baseline up to 24–52 weeks as compared to darbepoetin alpha (low certainty evidence). Similar results were reported in enarodustat, vadadustat, and roxadustat (very low certainty evidence). Molidustat reduced the hemoglobin levels from baseline up to 36 weeks as compared to darbepoetin alpha (very low certainty). Evidence from the existing studies was commonly of low to very low certainty. Included studies reported high risks of bias and serious impression. Trials were commonly open label leading to high risks of performance and detection biases.
There was paucity of studies evaluating clinically important outcomes like the progression to ESKD, patients requiring blood transfusion, MACE, fatigue, and QoL. Most of the studies were on a small sample size with limited follow-up time and pharmaceutical companies funded. Specifically, multicentric nonindustry research funded trials with adequate sample size; specifically, the South Asian countries should be prioritized to provide evidence on HIF-PHIs therapeutic effect on NDD-CKD patients. Robust Phase IV studies in approved markets are also required to establish long-term safety and risk-benefit ratio. Cost-benefit analysis should be done to understand the relative cost of HIF-PHIs with ESAs.
Our review was conducted according to a priori registered protocol. We used the standard Cochrane methods to conduct this review. A comprehensive search strategy with no publication date filter was used. All the steps were independently undertaken by at least two authors. While other systematic reviews have pooled different HIF stabilizers agents, our review synthesized evidence on individual HIF-PHI agents to assess their efficacy and safety to be used as alternatives to ESAs in NDD-CKD patients with anemia.
Evidence is scanty to inform decision-making and clinical practice. HIF-PHIs have uncertain effects on adverse events and MACE; hence, more trials are needed to assess the safety of these drugs.
Our meta-analysis provides evidence on the use of HIF-PHIs as an alternative to ESAs in NDD-CKDs.
Financial support and sponsorship
The systematic review was done to support a guideline development work. The George Institute for Global Health India received an unrestricted institutional grant from Zydus Lifesciences Ltd. for the guideline development. The funder has no role in any part of the systematic review, including the decision to conduct it, methodology, or publish it.
Conflicts of interest
There are no conflicts of interest.
References
- Complications of progression of CKD. Adv Chronic Kidney Dis. 2011;18:400-5.
- [CrossRef] [PubMed] [Google Scholar]
- Iron deficiency in chronic kidney disease: Updates on pathophysiology, diagnosis, and treatment. J Am Soc Nephrol. 2020;31:456-68.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: A network meta-analysis. Cochrane Database Syst Rev. 2014;2014:CD010590.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The efficacy and safety of roxadustat for anemia in patients with chronic kidney disease: A meta-analysis. Nephrol Dial Transplant. 2021;36:1603-15.
- [CrossRef] [PubMed] [Google Scholar]
- Renal anemia: From incurable to curable. Am J Physiol Renal Physiol. 2013;305:F1239-48.
- [CrossRef] [PubMed] [Google Scholar]
- A new approach to treating renal anaemia. Nat Rev Nephrol. 2019;15:731-2.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- FibroGen Reports Third Quarter 2021 Financial Results. Global Newswire; 2021. 9th November 2021. 20th January, 2022. Available from: https://www.globenewswire.com/en/news-release/2021/11/09/2330835/33525/en/FibroGen-Reports-Third-Quarter-2021-Financial-Results.html [Last accessed on 2022 Jan 20]
- Desidustat: First approval. Drugs. 2022;82:1207-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors for Treatment of Anemia in Chronic Kidney Disease: Guidelines for South Asia. Indian J Nephrol. 2025;35:129-67.
- [CrossRef] [Google Scholar]
- Rayyan: A web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- RobotReviewer: Evaluation of a system for automatically assessing bias in clinical trials. Available from: https://vortext.systems/robotreviewer [Last accessed on 2022 Jan 20].
- The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019.
- The GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group; 2013.
- Desidustat in anemia due to non-dialysis-dependent chronic kidney disease: A phase 3 study (DREAM-ND) Am J Nephrol. 2022;53:352-60.
- [CrossRef] [PubMed] [Google Scholar]
- Daprodustat compared with epoetin beta pegol for anemia in Japanese patients not on dialysis: A 52-week randomized open-label phase 3 trial. Am J Nephrol. 2021;52:26-35.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Daprodustat for the treatment of anemia in patients not undergoing dialysis. N Engl J Med. 2021;385:2313-24.
- [CrossRef] [PubMed] [Google Scholar]
- A Phase 3 study of enarodustat in anemic patients with CKD not requiring dialysis: The symphony ND study. Kidney Int Rep. 2021;6:1840-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of molidustat in the treatment of anemia in CKD. Clin J Am Soc Nephrol. 2019;14:28-39.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Molidustat for renal anemia in nondialysis patients previously treated with erythropoiesis-stimulating agents: A randomized, open-label, phase 3 study. Am J Nephrol. 2021;52:884-93.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of molidustat for anemia in ESA-naive nondialysis patients: A randomized, phase 3 trial. Am J Nephrol. 2021;52:871-83.
- [CrossRef] [PubMed] [Google Scholar]
- Phase 3 study of roxadustat to treat anemia in non-dialysis-dependant CKD. Kidney Int Rep. 2021;6:1810-28.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Vadadustat in patients with anemia and non-dialysis-dependent CKD. N Engl J Med. 2021;384:1589-1600. Availabel from: https://doi.org/10.1056/NEJMoa2035938
- [CrossRef] [PubMed] [Google Scholar]
- Phase 3 randomized study comparing vadadustat with darbepoetin alfa for anemia in Japanese patients with nondialysis-dependent CKD. J Am Soc Nephrol. 2021;32:1779-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Daprodustat for anemia: A 24-week, open-label, randomized controlled trial in participants with chronic kidney disease. Clin Kidney J. 2019;12:129-38.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Roxadustat for the treatment of anaemia in chronic kidney disease patients not on dialysis: A Phase 3, randomized, open-label, active-controlled study (DOLOMITES) Nephrol Dial Transplant. 2021;36:1616-28.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








