Translate this page into:
IgA Nephropathy with Posterior Reversible Encephalopathy with Spinal Cord Involvement in a Young Male: A Case Report
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Posterior reversible encephalopathy syndrome (PRES) is a clinico-radiological phenomenon commonly associated with kidney diseases, especially chronic kidney disease. A consequence of endothelial dysfunction, PRES is usually associated with uncontrolled blood pressures and can rarely have atypical radiological findings involving the brain stem and spinal cord, called posterior reversible encephalopathy with spinal cord involvement (PRES-SCI). These atypical features may be confused with other etiologies causing a delay in diagnosis and management. We describe a young male patient who presented with neurological symptoms suggestive of PRES; however, the atypical radiological findings along with concomitant rapidly progressive glomerulonephritis led to a diagnostic dilemma. Repeat neuro-imaging after appropriate blood pressure control showed disappearance of the lesions confirming the diagnosis of PRES-SCI, and kidney biopsy showed advanced IgA nephropathy. Knowledge of atypical features of PRES is crucial amongst nephrologists as it is a common association with kidney disease and prompt identification and management avoid irreversible sequelae and unnecessary investigations.
Keywords
Atypical
IgA nephropathy
PRES
rapidly progressive glomerulonephritis
Introduction
Posterior reversible encephalopathy syndrome (PRES), first described by Hinchey et al.,[1] is characterized by acute cerebral endotheliopathy leading to disruption of the blood–brain barrier and vasogenic edema. PRES occurs in all age groups; however, it is commoner in younger and middle-aged adults with a female preponderance.[2] As the name suggests, it is reversible and commonly involves areas supplied by the posterior circulation. Patients with hypertension, chronic kidney diseases, dialysis dependency, pregnancy, sepsis. and those on immunosuppressive drugs are known to develop PRES.[134] PRES is attributed to one or more factors, such as high blood pressure, drugs, toxins, malignancies, cytokines, and pregnancy, all of which cause endothelial dysfunction and cerebral hypoperfusion.[5] Blood pressure fluctuations are seen in 61 to 80% of the patients with PRES.[2] The clinical manifestations may vary depending upon the area of involvement, parieto-occipital lobes (typical regions), frontal lobe, cerebellum, basal ganglia, and spinal cord (atypical regions). Permanent neurological sequelae occur in up to one-quarter of patients not appropriately treated.[6] As it is commonly encountered in association with kidney diseases, knowledge of the spectrum of presentation of PRES would help clinicians in managing patients with kidney diseases.
Case Report
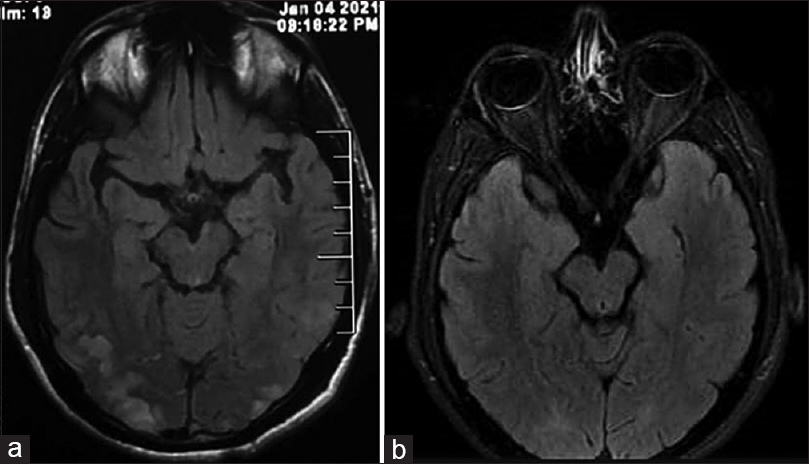
A 28-year-old-male patient without prior comorbidities, presented with complaints of holo-cranial headache, nausea, and vomiting for 7 days. Following the seventh day of headache, he developed two episodes of generalized tonic–clonic seizures lasting for 5 min each. He complained of blurred vision and transient shooting pain in both arms before the seizure onset. Urine output was noted to be reduced for a week before the current presentation. There was no history of fever, altered behavior, or weakness of limbs. There was no history of recreational drug intake, recent medication intake, or any infections in the recent past. There was no history of shortness of breath or chest pain and he denied bowel or bladder disturbances. Examination revealed an average built individual with a blood pressure of 190/100 mmHg, pulse rate of 90/min, and respiratory rate of 18/min. He was drowsy but arousable. Pulses were equally felt in all four limbs and there were no bruits. Fundus showed bilateral grade III hypertensive retinopathy changes. Cardiovascular and respiratory examinations were normal. There were no focal neurological deficits. The sensory and cerebellar examination was normal. Laboratory reports showed a hemoglobin of 11.0 g/L, total leucocyte count of 11 × 109/L, and platelet count of 220 × 109/L. Blood urea was 132 mg/dL, serum creatinine was 6.55 mg/dL. Aspartate and alanine transaminase levels were 56 IU/L and 49 IU/L, respectively. Urine examination showed 2+ albumin, 8–10 red blood cells (RBCs)/hpf. Ultrasonography of the kidneys showed bilateral normal-sized kidneys with increased echogenicity. Magnetic resonance imaging (MRI) of the brain and spine was done, which on T2 image showed changes involving mid-sagittal sections of the cervical spine having long segment signal changes from C2 to C7 levels [Figure 1a]. T2 fluid-attenuated inversion recovery (FLAIR) axial sections of the brain showed subcortical hyperintensities in the bilateral parieto-occipital region [Figure 1b]. Diffusion restriction or micro-hemorrhages were absent. The optic nerve and optic sheath were uninvolved. Magnetic resonance angiography did not show any vascular attenuation. Cerebrospinal fluid (CSF) workup was normal. Echocardiography showed concentric left ventricular hypertrophy. Kidney biopsy showed mesangial-proliferative glomerulonephritis with predominant fibrous crescents in 4/11 glomeruli and severe interstitial fibrosis and tubular atrophy of 60%. Immunofluorescence confirmed the diagnosis of IgAN. Auto-antibody panel, to investigate the possibility of myelitis in light of the MRI findings, including anti-aquaporin-4 antibody, anti-myelin oligodendrocyte glycoprotein antibody, anti-nuclear antibody, and anti-neutrophilic cytoplasmic-antibody (ANCA) titers, was normal. Advanced kidney dysfunction, short duration of symptoms, and investigations suggested rapidly progressive glomerulonephritis (RPGN). The presence of seizures, headache, and altered sensorium along with hypertensive crisis was suggestive of PRES. However, extensive hyperintensities along the spinal cord led to the consideration of alternative differential diagnoses. The following working diagnoses were considered: 1. autoimmune myelitis and glomerulonephritis are explained by one etiology- systemic vasculitis such as ANCA-associated vasculitis, 2. autoimmune myelitis and glomerulonephritis are explained by different etiologies such as neuromyelitis optica-spectrum disorder (NMO-SD) and IgAN, NMO-SD with ANCA-associated vasculitis, 3. glomerulonephritis and reversible cerebral vasoconstriction syndrome (RCVS), 4. glomerulonephritis and atypical PRES. His blood pressures were reduced by 25% over 1 h with labetalol infusion, and to <150/90 mmHg over the next 4 days [Figure 2]. Immunosuppressive medications were not given for treating kidney dysfunction due to advanced chronicity on the biopsy. A repeat MRI after 10 days from the insult revealed resolution of the chord changes [Figure 3a] and a significant reduction in the subcortical hyperintensities in parietal-occipital regions [Figure 3b]. There were no similar episodes noted in the follow-up of 12 months. His vision improved to 6/6 with the control of hypertension. Though blood pressures were well controlled, his kidney function declined progressively to end-stage kidney disease over the next 10 months.

- (a) T2 mid-sagittal sections of the cervical spine showing long-segment signal changes from C2 to C7 levels. (b) T2 FLAIR axial sections of the brain showing subcortical hyperintensities in the bilateral parieto-occipital region
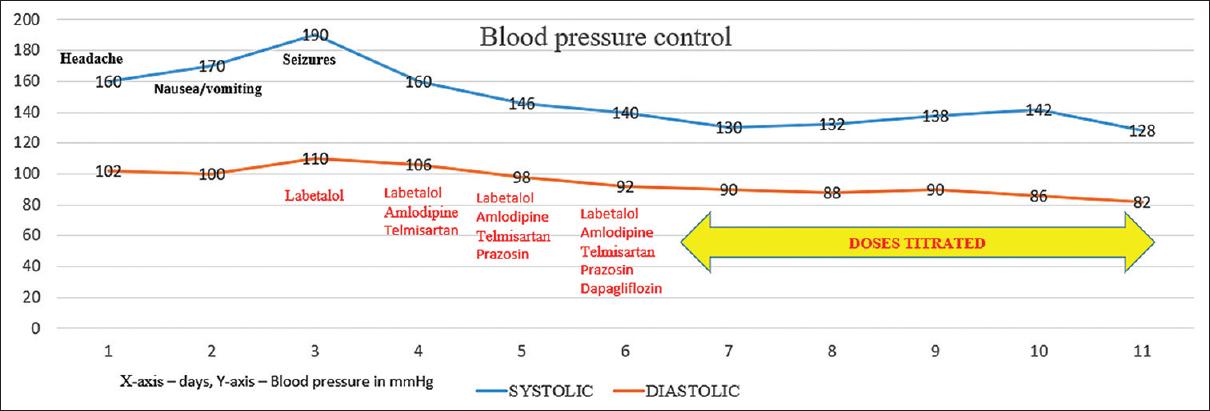
- Timeline of blood pressure reduction (blood pressure in mmHg on the x-axis, time in days on the y-axis)
- (a) Follow-up images done after 10 days show complete resolution of the cord findings. (b) Follow-up images done after 10 days show a significant reduction in the sub-cortical hyperintensities
Discussion
We report a young male with severe kidney dysfunction, hypertensive crisis, and neurological symptoms in the form of seizures and blurring of vision. Although PRES was an initial working diagnosis, neuro-imaging suggestive of extensive spinal cord involvement, not typical of PRES, led to uncertainty. An alternative possibility of systemic autoimmune disease such as ANCA-associated vasculitis was considered in view of concomitant RPGN and spinal cord hyperintensities, which could possibly reflect myelitis. However, kidney biopsy, done later, revealed IgAN, and the imaging findings of hyperintensities disappeared with intensive blood pressure control, consistent with PRES with spinal cord involvement (PRES-SCI).
PRES is associated with kidney dysfunction in 55% of patients.[6] IgA nephropathy, particularly in patients with known chronic kidney disease due to IgA nephropathy, has been described in association with PRES.[3] It is still unknown whether kidney dysfunction is a causative factor or concurrent presence of other factors is responsible for the causation of PRES. Our patient presented with atypical PRES at the time of the first presentation as RPGN due to IgAN. It is crucial to be aware of findings suggestive of atypical PRES, especially in the background of RPGN as glucocorticoid pulses, typically administered for RPGN, are thought to precipitate and worsen PRES.[7] Prevention and treatment of PRES are both pivotal in avoiding permanent neurological damage in patients with RPGN.
Clinical symptoms associated with PRES include encephalopathy, seizures, headache, visual disturbances, focal neurological deficits, and rarely status epilepticus. A diagnosis of PRES is usually aided by MRI. Classical radiological findings of PRES include posterior circulation predominant vasogenic edema showing as symmetrical lesions involving bilateral parietooccipital and precentral region subcortical white matter. It can be differentiated from ischemic lesions usually by lack of diffusion restriction and arterial territorial distribution. Diffusion restriction might be rarely seen in PRES; however, they are usually punctate contrary to confluent cytotoxic edema of infarcts.[4] Frontal lobes and temporal lobes are involved in 68% and 40% of the cases, respectively.[4] The brainstem and cerebellum can be involved in up to 30% of cases.[6] Our patient had symmetrical involvement of the parieto-occipital lobes with a longitudinally extensive lesion in the spinal cord, which created an initial diagnostic dilemma. PRES-SCI is important; however, a rare subtype of PRES. It presents as confluent longitudinally extensive spinal cord signal changes commonly involving the cervicodorsal cord. Pathology of vasogenic edema being the same, there is neither significant cord expansion nor enhancement. It may or may not be associated with intracranial PRES type lesions and classically associated with better prognosis. RCVS is an important differential diagnosis for PRES clinically. The presence of classical imaging findings such as multifocal infarcts, sulcal subarachnoid hemorrhage, and multifocal segmental narrowing of intracranial vessels in RCVS differentiate it from PRES; however, they often coexist.[8] Other imaging differentials for PRES-SCI include NMO-SD, and infective or immune-mediated transverse myelitis, which are differentiated by clinico-radiological correlation. We conducted additional investigations including an invasive procedure such as a spinal tap. The absence of significant clinical symptoms of myelitis and reversal of imaging findings differentiated those entities from PRES in our patient.[9] Diagnosis of PRES-SCI was confirmed when spinal lesions disappeared with the control of hypertension. Of note, blood pressure reduction must be done in a step-wise manner to avoid cerebral and retinal ischemia from overjealous blood pressure control.
Conclusion
PRES is common in patients with kidney disease. Atypical radiological findings are being increasingly identified with frequently used and advanced neuro-imaging techniques. Early recognition of atypical PRES is crucial for physicians managing kidney diseases to avoid unnecessary investigations and irreversible end-organ damage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500.
- [Google Scholar]
- Posterior reversible encephalopathy syndrome in kidney disease. Kidney Int Rep. 2017;3:502-7.
- [Google Scholar]
- Posterior reversible encephalopathy syndrome (PRES): Features on CT and MR imaging. Diagn Interv Imaging. 2013;94:45-52.
- [Google Scholar]
- Posterior reversible encephalopathy syndrome (PRES): Pathophysiology and neuro-imaging. Front Neurol. 2020;11:463.
- [Google Scholar]
- Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914-25.
- [Google Scholar]
- Corticosteroid therapy and severity of vasogenic edema in posterior reversible encephalopathy syndrome. J Neurol Sci. 2017;380:11-5.
- [Google Scholar]
- Reversible cerebral vasoconstriction syndrome presenting asconvexity subarachnoid hemorrhage and posterior reversible encephalopathy syndrome during postpartum: A case report and literature review. Neurol Asia. 2020;25:53-7.
- [Google Scholar]
- Posterior reversible encephalopathy syndrome with spinal cord involvement. Neurology. 2014;83:2002-6.
- [Google Scholar]







