Translate this page into:
Impact of Angiotensin Receptor Blockers (ARB) versus Other Antihypertensive Medication on Blood Pressure in Patients on Dialysis: A Meta-Analysis
Corresponding author: Reenaa Mohan, Department of Community Medicine, Sri Manakula Vinayagar Medical College and Hospital, Puducherry, India. E-mail: reenaamohan1406@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Devi DS K, Mary JJF, Mohan R, Gavlasova D, Kalaiselvan G, Kathiravan E, et al. Impact of Angiotensin Receptor Blockers (ARB) versus Other Antihypertensive Medication on Blood Pressure in Patients on Dialysis: A Meta-Analysis. Indian J Nephrol. 2024;34:431-41. doi: 10.25259/ijn_365_23
Abstract
Introduction
Hypertension is an important factor driving mortality among dialysis patients. Angiotensin-II receptor blocker (ARB) has been effective similarly to angiotensin-converting enzymes (ACEs) but with a low incidence of side effects.
Methodology
The meta-analysis included all published studies that investigated the effect of ARB on the hypertension in adult dialysis patients (≥18 years). Data extraction was guided by a predetermined checklist. Data sources of the retrieved studies were PubMed, MEDLINE, ScienceDirect, SCOPUS, Cochrane, Web of knowledge, and Google Scholar were systematically searched until February 2023. Using the RevMan 5 software, the mean difference for systolic and diastolic BP (SBP and DBP) and the risk ratio (RR) of the adverse events (AEs) were pooled from the selected studies. The random-effects model was used to compare the difference in the pre-and post-dialysis of the SBP and DBP. Data analyses were performed from December 2022 to February 2023. The primary outcome was the reduction in SBP and DBP in dialysis hypertensive patients who were on anti-hypertensive agents, and the secondary outcome was assessment of AE associated with the drug after dialysis (PROSPERO Registration: CRD42022355369).
Results
The initial search yielded 1,679 records, of which 84 studies underwent full-text evaluation, which identified 13 studies and 1,462 patients. The pooled standard MD for losartan with other anti-hypertensive agents, where the pre-dialysis SBP was 0.17 (95% confidence interval [CI]: −0.21–0.55) and the post-dialysis was 0.35 (95% CI: −0.17–1.02); yet, both are statistically non-significant, implies that there was no difference between Losartan and ARB drugs regarding the effect on the SBP. Diastolic BP for predialysis was −0.01 (95% CI: −0.65–0.63) and post-dialysis was 0.03 (95% CI: −0.24−0.30) and statistically non-significant. AEs by the ARB agents were lower compared to other anti-antihypertensive agents (relative risk [RR]: 1.01; 95% CI: 0.59–1.75) and statistically non-significant.
Conclusion
This systematic review and meta-analysis of RCT demonstrated that ARB and other anti-hypertensive medications had similar impacts on the treatment of hypertension.
Keywords
Angiotensin receptor blocker
Antihypertensive agents
Diastolic blood pressure
Hemodialysis
Losartan
Post-dialysis
Systolic blood pressure
Introduction
For patients with end-stage renal disease (ESRD), hemodialysis is a life-sustaining therapy.1 Yet, a documented consequence of hemodialysis is the potential for blood pressure (BP) to shift frequently both during and in between treatments.1 Large variability in BP measurements during hemodialysis is a risk factor for mortality.2 In general, BP is poorly controlled in patients on dialysis.3 The main causes of mortality among these patients under hemodialysis are cardiovascular (CV) complications, with high BP being an important risk factor.4
According to the National Kidney Foundation Kidney Disease (NKFKD) Outcomes Quality Initiative guidelines (2004), hypertension (HTN) in hemodialysis patients is diagnosed when pre-dialysis BP is >140/90 mmHg or when post-dialysis BP is >130/80 mmHg.5 The intradialytic HTN is defined as “an increase in BP during or immediately after hemodialysis which results in post-dialysis HTN.” The potential pathophysiologic mechanisms for such intradialytic HTN varies include volume overload, sympathetic overactivity, activation of the renin–angiotensin–aldosterone system, endothelial dysfunction, dialysis-specific factors, medications (erythropoietin-stimulating agents) and vascular stiffness.6,7 Given the established pathophysiology, non-pharmacologic therapies, such as dietary sodium restriction, improved salt removal with dialysis, and probing dry weight is the considered first steps in achieving BP control in these patients.7-9 Though these therapeutic approaches were considered to be challenging, as several factors contribute to it.9,10
A pooled analysis of randomized studies revealed that reducing BP lowers CV morbidity and mortality in dialysis patients, especially in those with high BP.7,8,11,12 Among the antihypertensive agents, losartan, a selective angiotensin-II receptor blocker (ARB), has shown an efficacy similar to angiotensin-converting enzyme inhibitors (ACEIs), with a low incidence of side effects13 and also well tolerated by patients with chronic kidney disease.14 Additionally, it helps prevent pathological CV remodeling by having an inhibitory influence on ambulatory short-term BP variability during the night.15 The most common treatment-related adverse events (AEs) were hypotension during or after dialysis and a mild increase in potassium levels.16,17 Studies showed that overall mortality was 1% when losartan was continued for 6 months.17 However, a “one-size-fits-all” approach for BP management may not be appropriate for all patients.18 The adjusted increased risk of death at 2 years was estimated to be 6% for every 10 mmHg increase in systolic blood pressure (SBP) during hemodialysis.19
Despite these advancements in this field, there is currently no comprehensive review of the effectiveness of ARBs such as Losartan in comparison with other anti-hypertensive drugs. This systematic review and meta-analysis aims to assess the overall outcomes of the effectiveness of ARBs such as Losartan and its impact on BP in patients on dialysis.
Methods
This protocol was prospectively registered with PROSPERO and conducted with the requirements of the reporting rules in the “Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines”.20 Because this work is a systematic review, the heterogeneity was present within the acceptable range, meta-analysis was performed.
Eligibility criteria
Criteria for included studies were defined as adults aged ≥ 18 years who were on dialysis. The full eligibility criteria are available in the eMethod 1 in Supplementary File 1. Studies were included if they studied adults of both sexes aged ≥ 18 years undergoing hemodialysis or dialysis receiving antihypertensive medication; were randomized controlled trials (RCTs) with individual or cluster-type, observational studies, assessed the effects of ARB and other antihypertensive agents (calcium channel blocker [CCB], alpha and beta blocker, diuretic) or placebo.
Search strategy
The electronic retrieval methods were adopted for the literature retrieval. A comprehensive and systematic research review using a combination of Medical Subject Headings (MeSH), controlled vocabulary, and keywords were conducted through various databases including PubMed, MEDLINE, ScienceDirect, SCOPUS, Cochrane, Web of Knowledge, and Google Scholar for studies until 2023. The full search strategy is available in the eMethod 2 in Supplementary File 1. Furthermore, a manual search of a reference list of primary studies was conducted from the selected topics, and relevant studies were included in the review and analysis.
Study selection
The search results were uploaded into the online systematic review program Covidence to.21,22 A two-stage screening process was conducted. Three independent authors (K.D, R.M, and J.J) performed the literature search and screened the title, abstract, and keywords. Screening of abstract and full text was performed independently by three authors (K.D, R.M, and J.J) to select the studies that satisfied the eligibility criteria. Any disagreements or discordances present were resolved either through consensus or consultation with the fourth author (J.F). If conflicts arose between reviewers, the fifth reviewer (K.D.H) moderated a discussion to come to a joint decision.
Data extraction and management
The relevant study characteristics were extracted by the first and co-author independently related to outcome measures. Data extraction was guided by a predetermined checklist with the first author’s last name, published year, the total sample size, gender, study design, duration of intervention, participants’ age, baseline and end-line BP (both systole [SBP and diastole [DBP]), type of antihypertensive (ACEI and ARB), type of control (placebo or other antihypertensives), and finally, number of AE were extracted [Table 1].
The second author (J.J.) transferred the obtained data into the software Review Manager (RevMan_5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).23 Data entry was double-checked for correct entry by the second author (J.J.) through a comparison of the data presented in the review and included in the reports.
Outcome measure for the study
The primary outcome was a reduction in SBP and DBP in dialysis hypertensive patients, and the secondary outcome was the assessment of AE associated with the drug.
Quality assessment
The National Institute of Health (NIH) study assessment quality tool24 was used to assess the risk of bias, and the quality review process was monitored.23 Each article was categorized as follows: “low-risk,” “moderate-risk,” or “high-risk” of bias [eTable 1 in Supplementary File 2].
Statistical analysis
A comprehensive qualitative analysis was made. Quantitative meta-analysis, of binomial data was performed using RevMan_5.3.23 When studies reported multiple arms in a single trial, only the relevant arms were included in the analysis. Due to heterogeneity among studies, a logistic-normal-random-effect model was conducted. The 95% confidence interval (CI) was performed for study-specific and overall pooled prevalence, respectively. To assess the heterogeny, I2 statistics were used. Significant heterogeny was considered if P value <0.05 or I2 > 50% among the studies.
Subgroup analysis was performed to assess the heterogeneity and potential confounding for studies. Study-specific and pooled estimates were graphically represented through forest plots for both combined and subgroup analyses. Publication bias was assessed and graphically represented by a funnel plot, and the asymmetry of the plot was tested using Egger’s test. Sensitivity analysis was performed to assess the reliability of the estimate obtained in the meta-analysis.
Results
Study selection and characteristics
A total of 1,679 studies were initially retrieved following the removal of duplicates. Of those, 15 studies met the inclusion criteria. However, 2 of the 15 studies were presented with already published patient information and were removed. Therefore, 13 studies were ultimately included for the qualitative and quantitative analyses [Table 1].15,25-36
| First Author | Year | Objective | Study duration | Study design | Intervention drug | Control drug | Results | Major limitation of the study | Type of dialysis | Complications >20% of n (even if anyone drug) |
|---|---|---|---|---|---|---|---|---|---|---|
| Aftab RA et al.25 | 2017 | To assess the effectiveness of Losartan in reducing BP among post-dialysis euvolemic patients and assessing their survival trends | 12 months | Multicentric, prospective, randomised, parallel design, single-blind trial | Losartan | Other antihypertensive drugs | BP Improved, Losartan 50 mg achieve an overall significant decline in blood pressure among post-dialysis euvolemic hypertensive patients | Small sample size, region specific population, | Hemodialysis | Events such as hyperkalaemia, cough, dizziness, and dyspepsia |
| Bikos A et al.28 | 2018 | To evaluate the effects of a single or weekly administration of nebivolol and irbesartan on peri-dialytic, intradialytic, and ambulatory BP in patients with intradialytic hypertension. | 3 months | Single-blind randomized-cross-over study | Irbesartan | Nebivolol | BP improved. Nebivolol was slightly more effective than irbesartan in reducing BP during weekly administration | Small sample size, short duration | Hemodialysis | Not mentioned |
| Huber M et al.29 | 2013 | To study the effect of telmisartan compared to placebo on top of a standard antihypertensive medication without treatment of other RAS blockers in HD patients. | 18 weeks | Placebo-controlled, double-blinded, cross-over randomization study | Telmisartan | Placebo | No average significant BP influence of telmisartan was observed compared to placebo. The latter may be due to a large inter-individual variability of BP responses. | Small sample size, Complicated standardization of concomitant medication and other confounding factors, | Hemodialysis | On telmisartan - leukocyte count was lowered |
| Ichihara A et al.30 | 2005 | To determine the low-dose effects of the ARB losartan and the angiotensin-converting enzyme inhibitor trandolapril on PWV, which predicts cardiovascular morbidity and mortality in hemodialysis patients. | 12 months | Multicentre, randomized, prospective study | Losartan | Trandolapril | In hemodialysis patients, trandolapril is as effective as losartan in decreasing PWV independent of its depressor effect and in suppressing elevated IDL-C levels | Less sample size, no blinding | Hemodialysis | Not mentioned |
| Peters CD et al.26 | 2015 | To describe central hemodynamics during dialysis newly started HD patients randomized to ARB (irbesartan) or placebo, aiming at a pre-dialytic SBP target of 140mmHg in both groups. | 12 months | Randomized Double-Blind Placebo-Controlled Trial | Irbesartan | Placebo | Over the one-year period, pre-dialytic SBP decreased significantly, but similarly in both groups. | Not mentioned | Hemodialysis | Not mentioned |
| Shigenaga A et al.33 | 2009 | To examine whether addition of an ARB, candesartan, or valsartan, to conventional antihypertensive treatment could improve BP in HTN patients on peritoneal dialysis | 6 months | Randomised controlled trial | Valsartan | Other than ACE inhibitors | Both showed similarly controlled 24-hour ambulatory BP values in hypertensive patients on peritoneal dialysis. ARB treatment is beneficial for the suppression of pathological CV remodelling with a decrease in BP variability. | Small sample size, peritoneal function not mentioned, norepinephrine level not mentioned, short duration. | Peritoneal dialysis | Not mentioned |
| Suzuki H et al.34 | 2008 | To evaluate the efficacy of long-term use of ARBs on CV outcomes in patients undergoing HD | 3 years | Prospective, controlled, randomized, open label trial | Losartan | Enalapril | There were 93 fatal or nonfatal CVD events (52%); 34 (19%) in the ARB group and 59 (33%) in the non-ARB group. | Small sample size, ARB were analysed as the group not as individual drugs, choice of the ARB was dependent based on the patients | Hemodialysis | |
| Suzuki H et al.35 | 2004 | To assess the effects of an ACE inhibitor, an AT1 antagonist and their combination on the regression of LVH in diabetic patients on dialysis therapy. | 1 year | Randomized controlled trial | Losartan | Enalapril | BP improved overall. An initial steady in blood pressure decline was observed for up to 6 months followed by maintenance and then a fall toward the end of the study. | The therapeutic window for intervention to prevent progressive cardiac enlargement | Hemodialysis | Intolerable cough |
| Peters CD et al.26 | 2015 | To describe central hemodynamics during dialysis newly started HD patients randomized to ARB (irbesartan) or placebo, aiming at a pre-dialytic SBP target of 140mmHg in both groups. | 12 months | Randomized Double-Blind Placebo-Controlled Trial | Irbesartan | Placebo | Over the one-year period, pre-dialytic SBP decreased significantly, but similarly in both groups. | Not mentioned | Hemodialysis | Not mentioned |
| Shigenaga A et al.33 | 2009 | To examine whether addition of an ARB, candesartan, or valsartan, to conventional antihypertensive treatment could improve BP in HTN patients on peritoneal dialysis | 6 months | Randomised controlled trial | Valsartan | Other than ACE inhibitors | Both showed similarly controlled 24-hour ambulatory BP values in hypertensive patients on peritoneal dialysis. ARB treatment is beneficial for the suppression of pathological CV remodelling with a decrease in BP variability. | Small sample size, peritoneal function not mentioned, norepinephrine level not mentioned, short duration. | Peritoneal dialysis | Not mentioned |
| Suzuki H et al.34 | 2008 | To evaluate the efficacy of long-term use of ARBs on CV outcomes in patients undergoing HD | 3 years | Prospective, controlled, randomized, open label trial | Losartan | Enalapril | There were 93 fatal or nonfatal CVD events (52%); 34 (19%) in the ARB group and 59 (33%) in the non-ARB group. | Small sample size, ARB were analysed as the group not as individual drugs, choice of the ARB was dependent based on the patients | Hemodialysis | |
| Suzuki H et al.35 | 2004 | To assess the effects of an ACE inhibitor, an AT1 antagonist and their combination on the regression of LVH in diabetic patients on dialysis therapy. | 1 year | Randomized controlled trial | Losartan | Enalapril | BP improved overall. An initial steady in blood pressure decline was observed for up to 6 months followed by maintenance and then a fall toward the end of the study. | The therapeutic window for intervention to prevent progressive cardiac enlargement does not remain open much beyond 1 year, this study would not apply to diabetic patients who had already received dialysis therapy >1 year prior. | Hemodialysis | Intolerable cough |
| Takahashi A et al.27 | 2006 | To investigate whether candesartan, an ARB type I, reduces the incidence of CV events in Hemodialysis patients. | 3 years | Prospective, randomized, open blinded trial | Candesartan | Placebo | BP was not different between the two groups and no changes were noted in the either group during follow-up. Candesartan therapy significantly reduces CV events and mortality in patients on chronic maintenance hemodialysis, independent of their anti-hypertensive effect. | Small size, short duration | Hemodialysis | Heart failure |
BP: Blood pressure; CV: cardiovascular; ARB: Angiotensin receptor blocker; SBP: Systolic blood pressure; HD: Hemodialysis; HTN: Hypertension; PWV: pulse wave velocity; RAS: Renin angiotensin system; IDL c: Intermediate density lipid cholesterol; CKD: chronic kidney disease; AVF: Arterio venous fistula; ESRD: End stage renal disease; SBP: systolic blood pressure; LVH: left ventricular hypertrophy; ACE: Angiotensin converting enzyme; AT1: angiotensin 1 receptor; OSI: oxidative stress index.
Of the 13 studies, seven studies focused on Losartan, and the remaining studies focused on other ARBs. When using the NIH quality assessment tool, seven studies had a low risk of bias, two studies had a moderate risk of bias, and the rest of four studies had a high risk of bias [eTable 1 in Supplementary File 2].15,25-36 The PRISMA flowchart for the study selection is available in Figure 1. The major limitation was the small sample size in seven studies and the short duration in three studies. BP reductions were found to be similar in both groups in six studies. Hyperkalaemia was found in two studies in >20% of the study sample as a complication.

- Preferred reporting items for systematic review and meta-analysis protocols (PRISMA) flow diagram of the study selection process.
Characteristics of the patient and the ARB agents
From all 13 studies included, 745 patients in the intervention group and 717 patients in the control group who underwent hemodialysis were given antihypertensive agents.15,25-36 The mean age for the overall cohorts included in this study ranged from 27.8 to 71 years of age. Seven studies used losartan, two studies used irbesartan, whereas the remaining four studies each used telmisartan, olmesartan, valsartan, and candesartan. Similarly, as for the control group, three studies had placebo, whereas the remaining studies used other antihypertensive agents.15,25-36
The duration of the intervention was ranged from 18 weeks to 3 years. Similarly, as for the selection criteria for the study population, the patients were selected based on the period of anti-hypertensive drug started in the process of dialysis. Six studies included the intradialytic period,15,27-29,35,36 six studies comprised the post-dialysis period,25,26,30-32,34 and one study had values from the pre-dialysis period.33
Methodological quality of the included studies
The 13 studies were all RCTs with other antihypertensive agents or placebo as a control. These studies were published between 2003 and 2018 and performed in the hospital setting. 11 studies were of parallel group RCT,15,25-27,30-36 whereas the other two were cross-over trials.28,29 Among these, three trials were double-blinded,26,29,33 four single-blinded studies,25,27,28,34 whereas five studies did not report blinding [Table 1].
Effectiveness of ARB drug on SBP and DBP among patients undergoing haemodialysis
A meta-analysis of 13 eligible RCT studies involving 745 subjects received ARBs and the 717 controls received other anti-hypertensive agents and placebo. The random model effects showed the pooled mean difference (MD) between the pre-dialysis and post-dialysis BP of the ARB agents and the other antihypertensive agents. The pooled standard MD for the SBP among pre-dialysis was 0.11 [95% CI: −0.09–0.31], [Figure 2a] when compared to the post-dialysis were 0.35 (95% CI: −0.33–1.02), [Figure 2b] which does not favor the effect of ARB agent on SBP when compared with other anti-hypertensive agents. Heterogeneity was found regarding the use of the ARB agent for SBP management among the studies included in the analysis (I2: 63% and 97%, respectively). Similarly, the pooled standard MD for the DBP among the pre-dialysis was −0.08 (95% CI: −0.42–0.26) with considerable heterogeneity (I2: 88%) [Figure 3a] when compared with the post-dialysis as 0.11 (95% CI: −0.18–0.40) [Figure 3b] and 82% was the heterogeneity. Complications by the ARB agents were assessed and found that the pooled risk ratio was 1.01 (95% CI: 0.59–1.75) with a heterogeneity of 75% and statistically non-significant [Figure 4].
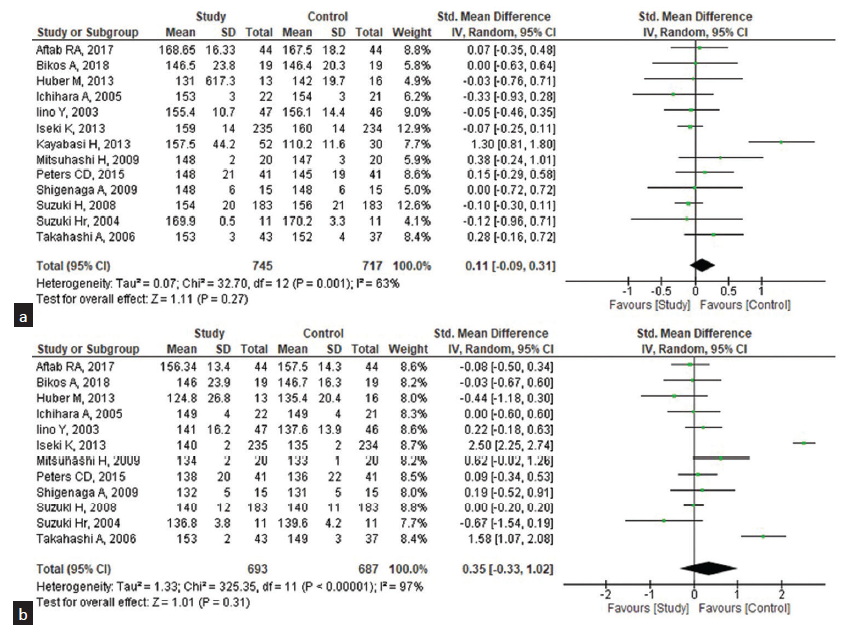
- Effectiveness of ARB drugs on the systolic blood pressure (SBP) among the patients undergoing pre- and post-hemodialysis (n = 13). (a) Pre-hemodialysis, (b) Post-hemodialysis. CI: Confidence interval, ARB: Angiotensin receptor blocker, SD: standard deviation.
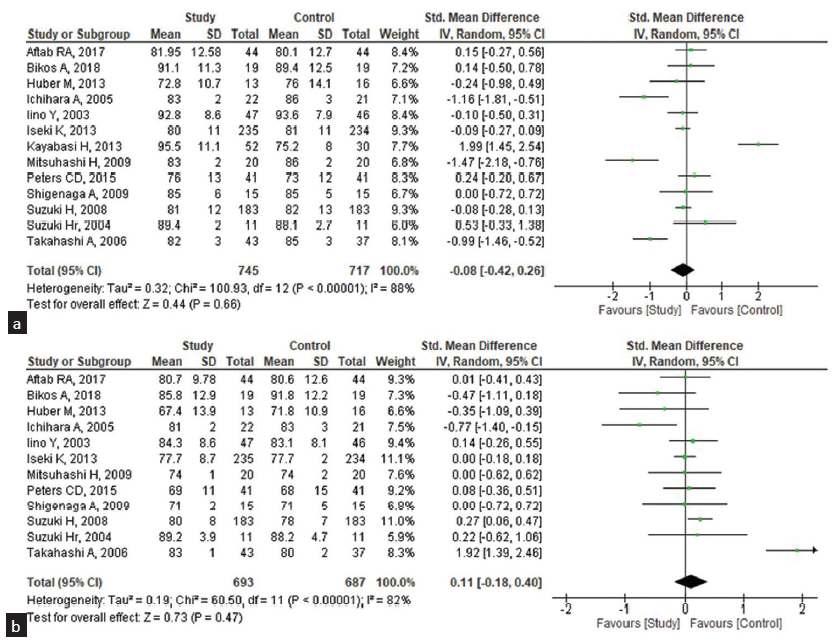
- Effectiveness of ARB drugs on the diastolic blood pressure (DBP) among the patients undergoing pre- and post-hemodialysis (n = 13). (a) Pre-hemodialysis, (b) Post-hemodialysis. CI: Confidence interval, ARB: Angiotensin receptor blocker, SD: standard deviation.
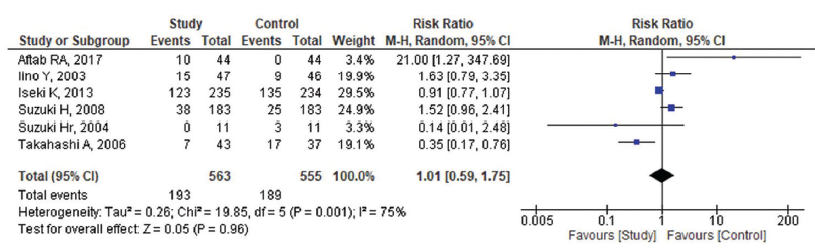
- Complications by angiotensin receptor blockers (ARB) agents among patients who underwent dialysis (n = 6). M-H: Mantel-Haenszel. CI: Confidence Interval.
Subgroup analysis
We performed subgroup analysis [Figures 5 and 6] to assess the heterogeneity and difference in the effect size of the ARB agent losartan across the types of ARB agents. For the pre-dialysis SBP, the pooled standard MD was 0.17 (95% CI: −0.21–0.55) and the post-dialysis was 0.35 (95% CI: −0.17– 1.02); yet, both were statistically non-significant, implying that there was no difference between losartan and ARB drugs where both had the same effect on the SBP. For the DBP, the pooled MD for pre-dialysis was −0.01 (95% CI: −0.65–0.63) and post-dialysis was 0.03 (95% CI: −0.24– 0.30) and statistically non-significant.
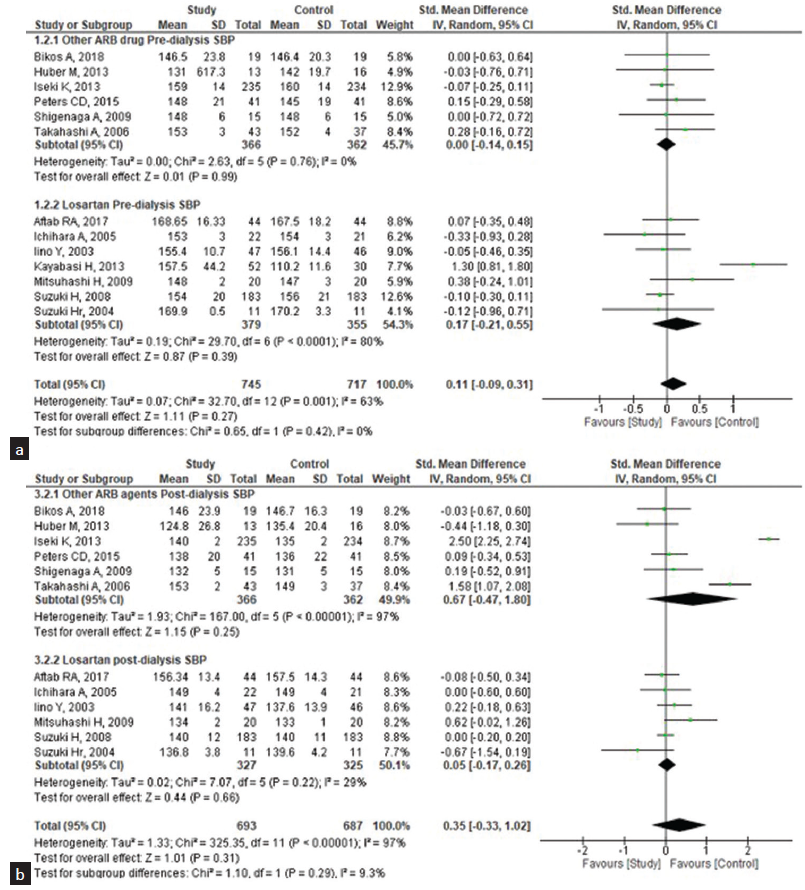
- Subgroup analysis of the effectiveness of losartan (study group) and other ARB drugs (control group) on systolic blood pressure (SBP) among the patients undergoing hemodialysis. (a) Pre-dialysis, (b) Post-dialysis. CI: Confidence interval, ARB: Angiotensin receptor blocker, SD: standard deviation.
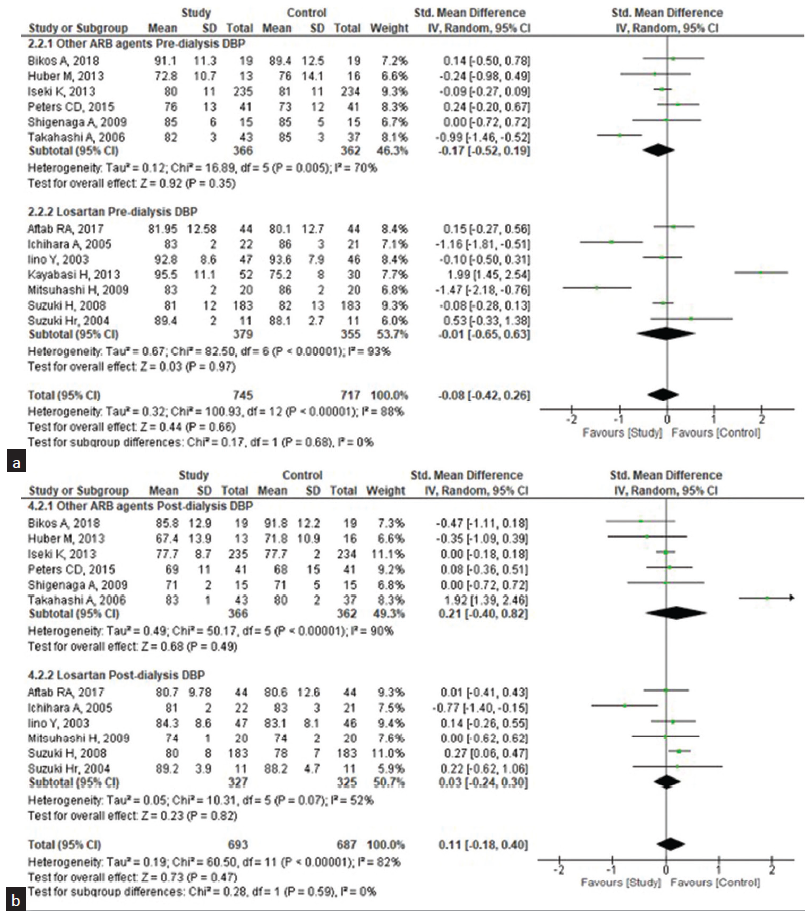
- Subgroup analysis of the effectiveness of losartan (study group) and other ARB drugs (control group) on diastolic blood pressure (DBP) among patients undergoing hemodialysis. (a) Pre-dialysis, (b) Post-dialysis. ARB: Angiotensin receptor blocker, CI: confidence interval, SD: standard deviation.
Publication bias and sensitivity analysis
Publication bias is presented in eFigures 1, and 2 in Supplementary File 2. We performed a sensitivity analysis eFigure 3 in Supplementary File 2 to assess the reliability of our results. We excluded studies with a study population of less than 30 and looked for any significant change in the results. It has been found the effect estimate for SBP increased from 1.01 to 1.37, with considerable heterogeneity (I2: 98%), whereas it was 0.73 to 1.82 (I2: 89%) for the DBP. The analysis found that those studies had an influence on other studies on the overall estimates obtained with minimum changes only.
Discussion
Hypertension in patients undergoing dialysis is associated with adverse clinical outcomes. Current clinical guidelines emphasize targeting SBP of <130 mmHg post-hemodialysis for chronic hemodialysis patients. The available literature on intradialytic hypertension shows a trend of SBP rise >10 mmHg from pre- to post-dialysis in the hypertensive range in at least four to six consecutive dialysis treatments37 showing that 8 to 30% of dialysis patients develop intradialytic hypertension. This newly developed hypertension is associated with a 2.5-fold increased risk for hospitalization and cardiovascular (CV) mortality.38 As the underlying pathophysiology is multifactorial, the treatment decision for intradialytic hypertension should be individualized.
Overall, we demonstrated that patients on hemodialysis who received ARB for hypertension in 13 studies included in this meta-analysis did not have better outcomes when compared with the use of other anti-hypertensive medications. In terms of SBP, our study findings are similar to Agarwal et al.’s7 study that showed that despite the use of two anti-hypertensive agents, on average, SBP is uncontrolled in the large majority of patients on drug therapy. In addition, these results were concordant with the report of Cannella et al.,39 who found that left ventricular hypertrophy (LVH) was caused by a high prevalence of inadequately or partially controlled hypertension. These studies implied varied effects of ARB on the BP, which might be due to the measurement of the BP at different periods of follow-up. Yet, these agents significantly reduced the mortality and the AE in patients with hemodialysis, with improved prognosis and survival. Additionally, we did not find any significant difference in the post-DBP (pooled MD: 1.21). This finding parallels a study that examined the effects of olmesartan on CV outcomes and mortality. Although the study observed a reduction in BP, it did not find any statistically significant differences in the measured outcomes.31 Intriguingly, numerous studies have demonstrated the positive impact of ARBs on DBP during follow-up periods, yielding significant favorable outcomes. ARB showed a significant reduction in DBP after intervention during the follow-up in 12 months,25 8 months,16 6 months,40 and 2 weeks duration.7 Added to that DBP was not an independent factor for the development of the CV event in dialysis patients.41 All these studies that showed positive results implied that the prolonged use of ARB resulted in a significant reduction of DBP with a significant reduction in CV mortality. Yet, assessment of high DBP is very unlikely to be isolated from SBP.17 Although high-quality evidence evaluating high DBP is limited due to its low incidence, we found no significant difference between ARB and other antihypertensive medications to reduce post-diastolic BP.
The existing literature showed that the effect of the ARB agent on hemodialysis patients helps maintain adequate BP and is well tolerated.26,27 However, as per our analysis, patients might require other antihypertensive agents to maintain appropriate BP.42
In the present study, we could observe studies with losartan had a significant influence on the SBP and DBP when compared to the other studies. The pooled MD and heterogeneity level of the losartan group for SBP was 0.21 (95% CI: −0.32, 0.73; I2: 79%), and for DBP was 2.06 (95% CI: −2.98, 7.10; I2: 86%); yet, it was statistically non-significant and considerable heterogeneity.
Studies showed that post-dialysis BP was observed to significantly decrease over time when hypertensive patients received losartan 50 mg.16 After 6 months, it significantly lowered SBP and DBP by about 10 and 5 mmHg, respectively. Also, the overall mortality was 1% when losartan was continued for 6 months.17
Yet, the other studies that used other groups of ARB agents for adequate SBP control also showed positive effects, when studies that used losartan were excluded. The pooled MD for SBP was 0.05 (95% CI: −0.23, 0.34) and for DBP was 0.24 (95% CI: −4.28, 4.77), respectively, and non-significant. They both showed very minimal heterogeneity (SBP I2: 0%; DBP I2: 52%) when compared with other antihypertensive agents. Studies have shown that losartan is also considered to have less AE.13-17,25 The results of this study support the role of losartan as an antihypertensive drug helping in maintaining HTN in hemodialysis patients although it is statistically non-significant in optimal control of hypertensive dialysis patients when compared to other antihypertensives.
Our systematic review has some limitations. As far as the lack of sufficient data with regard to losartan and other ARB agents, most studies published were observational studies and case series that concerned the exclusion criteria, and hence were susceptible to bias and confounding. The limited RCT that used ARB was only reviewed, which resulted in limited data. Due to the lack of proper random sequencing and allocation concealment, the effect of the ARB was potentially overestimated. Furthermore, due to the smaller sample size in each study and single-centered studies, it cannot be extrapolated to general studies.
Conclusion
Our review of completed clinical trials to date comparing ARBs and other antihypertensive medications, revealed that both exhibited promising outcomes. Losartan appears to facilitate optimal hypertension management in dialysis patients accompanied by minimal AEs. We propose further exploration involving the combination of additional antihypertensive agents with ARBs to ascertain the most effective BP management strategy.
Author contributions
Drs Devi DSK and Mary JJF had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Drs Devi DSK, Mary JJF, and Mohan R contributed equally. Drs Gavlasova D, Foppiani JA, Kalaiselvan G, Kathiravan, Devi MA, and Lin SJ were the co-seniors for the study. Devi DSK and Mohan R conceptualized the study and contributed to study design, data acquisition, manuscript writing. Mary JJF contributed to data acquisition, data analysis, manuscript writing, statistical analysis. Foppiani JA onceptualized the study and contributed to study design, manuscript editing, administrative, technical, or material support and supervision. Gavlasova D, Kalaiselvan G, Lin SJ helped with manuscript editing, administrative, technical, or material support and supervision. Kathiravan helped with manuscript writing and Saravanan V provided administrative, technical, or material support.
Conflicts of interest
There are no conflicts of interest.
References
- Mechanisms and treatment of intradialytic hypertension. Blood Purif. 2016;41:188-93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association of intradialytic blood pressure variability with increased all-cause and cardiovascular mortality in patients treated with long-term hemodialysis. Am J Kidney Dis. 2013;61:966-74.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence, Treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115:291-7.
- [CrossRef] [PubMed] [Google Scholar]
- Congestive heart failure in dialysis patients: Prevalence, incidence, prognosis and risk factors. Kidney Int. 1995;47:884-90.
- [CrossRef] [PubMed] [Google Scholar]
- K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 Suppl 1):S1-290.
- [CrossRef] [PubMed] [Google Scholar]
- Intradialytic hypertension: A less-recognized cardiovascular complication of hemodialysis. Am J Kidney Dis. 2010;55:580-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Assessment and management of hypertension in patients on dialysis. J Am Soc Nephrol JASN. 2014;25:1630-46.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Blood pressure in chronic kidney disease stage 5d-report from a kidney disease: Improving global outcomes controversies conference. Kidney Int. 2010;77:273-84.
- [CrossRef] [PubMed] [Google Scholar]
- Dry-w eight: A concept revisited in an effort to avoid medication-directed approaches for blood pressure control in hemodialysis patients. Clin J Am Soc Nephrol CJASN. 2010;5:1255-60.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Shorter delivered dialysis times associate with a higher and more difficult to treat blood pressure. Nephrol Dial Transplant. 2013;28:1562-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: A systematic review and meta-analysis of randomised controlled trials. Lancet Lond Engl. 2009;373:1009-15.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cardiovascular protection with antihypertensive drugs in dialysis patients: Systematic review and meta-analysis. Hypertens Dallas Tex 1979. 2009;53:860-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Safety and tolerability of losartan potassium, an angiotensin II receptor antagonist, compared with hydrochlorothiazide, atenolol, felodipine er, and angiotensin-converting enzyme inhibitors for the treatment of systemic hypertension. Am J Cardiol. 1995;75:793-5.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical safety and tolerability of losartan. Clin Ther. 1997;19:604-16. discussion 603
- [CrossRef] [PubMed] [Google Scholar]
- Effect of losartan on ambulatory short-term blood pressure variability and cardiovascular remodeling in hypertensive patients on hemodialysis. Atherosclerosis. 2009;207:186-90.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of losartan in the management of post-dialysis euvolemic hypertension (held-trial): A single-blind randomized control trial. Sci Rep. 2016;6:36592.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evaluation of the Losartan in Hemodialysis (ELHE) study. Kidney Int Suppl. 1998;68:S125-9.
- [CrossRef] [PubMed] [Google Scholar]
- Age, race, diabetes, blood pressure, and mortality among hemodialysis patients. J Am Soc Nephrol JASN. 2010;21:1970-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pharmacotherapy of hypertension in chronic dialysis patients. Clin J Am Soc Nephrol CJASN. 2016;11:2062-75.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Product review: Covidence (systematic review software) J Can Health Libr Assoc J Assoc Bibl Santé Can. 2014;35:68-71.
- [CrossRef] [PubMed] [Google Scholar]
- Covidence-better systematic review management. Covidence. Available from: https://www.covidence.org/. [Last accessed on 2022 Dec 04].
- RevMan 5. Available from: https://training.cochrane.org/online-learning/core-software/revman/revman-5-download. [Last accessed on 2022 Jul 19].
- Study Quality Assessment Tools. NHLBI, NIH. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. [Last accessed on 2022 Dec 03].
- Safety and efficacy of losartan 50 mg in reducing blood pressure among patients with post-dialysis euvolemic hypertension: A Randomized Control Trial. Sci Rep. 2017;7:17741.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- No significant effect of angiotensin II receptor blockade on intermediate cardiovascular end points in hemodialysis patients. Kidney Int. 2014;86:625-37.
- [CrossRef] [PubMed] [Google Scholar]
- Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis—a randomized study. Nephrol Dial Transplant. 2006;21:2507-12.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of nebivolol and irbesartan on postdialysis and ambulatory blood pressure in patients with intradialytic hypertension: A randomized cross-over study. J Hypertens. 2019;37:432-42.
- [CrossRef] [PubMed] [Google Scholar]
- Dialysis-associated hypertension treated with telmisartan – diatel: A pilot, placebo-controlled, cross-over, randomized trial. PLoS One. 2013;8:e79322.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Low doses of losartan and trandolapril improve arterial stiffness in hemodialysis patients. Am J kidney Dis. 2005;45:866-74.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of Angiotensin Receptor Blockade (ARB) on mortality and cardiovascular outcomes in patients with long-term haemodialysis: A randomized controlled trial. Nephrol Dial Transplant. 2013;28:1579-89.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of losartan on oxidative stress and inflammation in non-diabetic patients undergoing chronic hemodialysis. Eur Rev Med Pharmacol Sci. 2013;17:235-42.
- [PubMed] [Google Scholar]
- Effects of angiotensin II type 1 receptor blocker on blood pressure variability and cardiovascular remodeling in hypertensive patients on chronic peritoneal dialysis. Nephron Clin Pract. 2009;112:c31-40.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: An open-label randomized controlled trial. Am J Kidney Dis. 2008;52:501-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the effects of angiotensin receptor antagonist, angiotensin converting enzyme inhibitor, and their combination on regression of left ventricular hypertrophy of diabetes type 2 patients on recent onset hemodialysis therapy. Ther Apher Dial. 2004;8:320-7.
- [CrossRef] [PubMed] [Google Scholar]
- Interim evidence of the renoprotective effect of the angiotensin ii receptor antagonist losartan versus the calcium channel blocker amlodipine in patients with chronic kidney disease and hypertension: A report of the Japanese Losartan Therapy Intended for Global Renal Protection in Hypertensive Patients (JLIGHT) study. Clin Exp Nephrol. 2003;7:221-30.
- [CrossRef] [PubMed] [Google Scholar]
- Blood pressure and volume management in dialysis: conclusions from a kidney disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2020;97:861-76.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Management of intradialytic hypertension: The ongoing challenge. Semin Dial. 2006;19:141-5.
- [CrossRef] [PubMed] [Google Scholar]
- Inadequate diagnosis and therapy of arterial hypertension as causes of left ventricular hypertrophy in uremic dialysis patients. Kidney Int. 2000;58:260-8.
- [CrossRef] [PubMed] [Google Scholar]
- Angiotensin-converting enzyme inhibitors or angiotensin receptor blocker monotherapy retard deterioration of renal function in taiwanese chronic kidney disease population. Sci Rep. 2019;9:2694.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Low on-treatment diastolic blood pressure and cardiovascular outcome: a post-hoc analysis using nhlbi sprint research materials. Sci Rep. 2019;9:13070.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antihypertensive agents in hemodialysis patients: A Current Perspective. Semin Dial. 2010;23:290-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







