Translate this page into:
Indian TrANslational GlomerulonephrItis BioLogy nEtwork (I-TANGIBLE): Design and Methods
Address for correspondence: Prof. Vivekanand Jha, George Institute for Global Health, 308-09, Third Floor, Elegance Tower, Jasola District Centre, New Delhi – 110 025, India. E-mail: vjha@georgeinstitute.org.in
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Aim:
Primary glomerular disease accounts for one-sixth of all chronic kidney diseases (CKDs) in India. We remain limited in our ability to effectively treat these conditions because of lack of understanding of the disease mechanisms and lack of predictors to identify the clinical course and therapeutic responsiveness. We propose to develop a network of investigators in glomerular diseases, collect information in a systematic fashion to understand the clinical outcomes, answer translational research questions better, and identify and recruit patients for clinical trials.
Materials and Methods:
This is a prospective, observational study. The Indian TrANslational GlomerulonephrItis BioLogy nEtwork (I-TANGIBLE) cohort will enroll patients (>18 years) with biopsy-proven minimal change disease (MCD), focal segmental glomerulonephritis (FSGS), membranous nephropathy (MN), IgA nephropathy (IgAN), or membranoproliferative glomerulonephritis (MPGN) (immune complex- and complement-mediated), with first biopsy taken within 2 years of enrollment. Patients with estimated glomerular filtration (eGFR) rate <15 ml/min/1.73 m2 for >3 months at the time of screening, kidney transplant or bone marrow transplant recipients, patients with active malignancy, and patients with active hepatitis B/C replication or human immunodeficiency virus (HIV)-I/II will be excluded. Clinical details including history, medication history and details, and family history will be obtained. Consenting patient’s blood and urine samples will be collected and stored, aligned to their clinical follow-up.
Expected Outcomes:
The network will allow accurate ascertainment of disease burden of glomerular diseases across study sites, establishment of the treatment pattern of common glomerular diseases, investigation of medium- and long-term outcomes (remission, relapse, rate of eGFR decline), and building a suitable infrastructure to carry out clinical trials in primary glomerular disease.
Keywords
Focal segmental glomerulonephritis (FSGS)
longitudinal cohort
membranous nephropathy (MN)
membranoproliferative glomerulonephritis (MPGN)
minimal change disease (MCD)
Introduction
Glomerular diseases are the third most common cause of end-stage kidney disease (ESKD) worldwide. Membranous nephropathy (MN), focal segmental glomerulosclerosis (FSGS), immunoglobulin A nephropathy (IgAN), and membranoproliferative glomerulonephritis (MPGN) are the commonest histologically defined primary glomerulonephritis (GN) in adults that cause significant morbidity and account for 16% of ESKD in India,[12] second only to diabetic nephropathy of all the known causes of chronic kidney disease (CKD). However, a poor understanding of the disease pathogenesis and the lack of predictors in identifying the clinical course and therapeutic responses limit our ability to treat primary glomerular diseases effectively.
The existing morphology-based classification of glomerular diseases does not effectively contribute to understating the natural history of glomerular disease. There is an emerging need for a pathogenesis-based classification, as it will foster better understanding of disease and inform the development of novel therapeutic interventions. Recent epidemiologic, clinical, and basic research studies have implicated novel genetic[34] and environmental factors[56] in the pathogenesis of these disorders, which have begun to reveal underlying molecular pathways that can differentiate etiologically distinct subtypes.[6] The evolution of pathogenesis-based classification of MPGN[7] and MN[8] in the last few years facilitated accurate phenotyping of patients with these diseases, improved risk prediction, and paved the way for biological therapies that target specific points in the cascade of injury, maximizing the chances of favorable outcomes while minimizing treatment risk. Similarly, advances in understanding the genetic basis of FSGS[910] have led to the realization that those with the genetic disease should not be exposed to potentially toxic steroid therapy. Also, there is an increasing awareness to do a genetic screening in the younger population with this histology. Unfortunately, single-center studies with limited data are less likely to provide comprehensive assessment and testing for disease pathogenesis. Consolidating large-scale molecular and clinical data from a well-characterized cohort will accurately define patients by their underlying disease mechanism and reduce heterogeneity inherent in the morphology-based classification.
Recently, there have been initiatives from various countries to better understand the pathobiology of primary glomerular disease through glomerular disease networks [Table 1]. These groups aim to foster research and development of new therapies by creating a collaborative, precompetitive space for interactions among industry, government, patients, scientists, and the kidney care community. The most extensive network is the CureGN or Cure Glomerulonephropathy (www.CureGN.org).[11] Besides collecting clinical data, the network will develop a digital archive of kidney biopsy images and collect blood and urine specimens at study visits aligned with clinical care one to four times per year. Notably, the consortium is creating infrastructure to support scientific approaches to identify mechanistically distinct subgroups, identify accurate biomarkers of disease activity and progression, delineate disease-specific treatment targets, and inform future therapeutic trials. The Nephrotic Syndrome Study Network (NEPTUNE) is an ongoing observational incident cohort in North America,[12] with the aim to establish a collaborative, integrated, cost-effective investigational infrastructure to conduct clinical and translational research in FSGS, MCD, and MN. The Canadian Glomerulonephritis Registry (CGNR) and Translational Research Initiative is a multicentric (various centers across Canada) patient-centric database aimed at conducting clinical and molecular research in patients with primary glomerular disease.[13] The UK Rare Diseases Registry (RaDaR) program includes patients with 30 rare kidney diseases, but also patients with a primary glomerular disease like C3 glomerulopathy, IgAN, and primary MN. The objective of the aforementioned registry is to foster collaboration of patient groups and nephrologists to improve clinical care and research.[14] Similarly, the European PodoNet registry facilitates a comprehensive understanding of patients with steroid-resistant nephrotic syndrome, which will further serve as a platform for newer diagnostics and therapeutic decisions.[15]
| Consortium | Biosamples | Funding sources | Enrolling | Clinical phenotypes |
|---|---|---|---|---|
| CureGN | Yes | NIDDK | Yes | MCD, FSGS, MN, IgAN |
| NEPTUNE | Yes | NIDDK and NCATS | Yes | NS with MCD, FSGS, MN |
| UNC Glomerular Disease Collaborative Network | Yes | NIDDK P01 and philanthropy | Yes | All glomerular diseases |
| Canadian Glomerulonephritis Registry | Yes | Canadian Institutes of Health Research Kidney Foundation of Canada | IgAN, focal and segmental glomerulosclerosis, MCD, MN, C3 glomerulopathy, and membranoproliferative GN | |
| NephCure Accelerating Cures Institute | No | NephCure Kidney International | Yes | All forms of NS |
| Toronto Glomerulonephritis Registry | No | Divisional Fund University Health Network | Yes | All biopsy-proven forms of GN |
| British Columbia Glomerulonephritis Network and Registry | No | BC Provincial Renal Agency | Yes | Biopsy-proven GN |
| PodoNet (Europe) | Yes | EU via EURenOMICS Project | Yes | Steroid-resistant NS |
| NephroS—part of the United Kingdom Renal Rare Disease Registry | Yes | National Institute for Health Research, Kidney Research UK, Kids Kidney Research, industry partnerships | Yes | Idiopathic NS in children and adults |
| China-DiKip | Yes | National funding | Yes | Glomerular disease |
BC=British Columbia, CureGN=Cure Glomerulonephropathy, DiKip=China-Digital Kidney Pathology, EU=European Union, FSGS=focal segmental glomerulonephritis, GN=glomerulonephritis, IgAN=IgA nephropathy, MCD=minimal change disease, MGN=membranous glomerulonephritis, MN=membranous nephropathy, NCATS=National Center for Advancing Translational Sciences, NEPTUNE=Nephrotic Syndrome Study Network, NIDDK=National Institute of Diabetes and Digestive and Kidney diseases, NS=nephrotic syndrome, UNC=University of North Carolina
Why a South Asian registry is important?
Unique population characteristics can influence disease behavior including presentation and response to treatment. Unlike in other major geographies and ethnic populations, the clinical course and outcomes of glomerular diseases in South Asia have not been well studied. A few studies have documented the clinical and pathological patterns of MN, IgAN, C3 glomerulopathy, and postinfectious GN from India. These descriptions are limited to single-center studies with short follow-ups, but highlight a number of unique features. They include identification of high-risk genetic loci in MN,[16] identification of M-type phospholipase A2 receptor (PLA2R) as being important to MN pathogenesis, a marker of disease activity, and a therapeutic target, and complementary and alternative medicine-related MN.[1718192021] Glomerular Research And Clinical Experiments-IgA Nephropathy in Indian}s (GRACE-IGAI)[22] and other studies[2324] from India showed an overabundance of chronic lesions and lack of features indicating active disease in the kidney biopsy in IgAN. Arivazhagan et al.[25] showed poor therapeutic response to corticosteroids in patients with postinfectious GN, whereas Kumar et al. showed that infection had a role in predisposing to C3 glomerulopathy. Kumar et al.[26] also showed that there was a high prevalence of tubular atrophy and interstitial fibrosis in patients with C3 glomerulopathy, which portends a poor prognosis. The impact of other unique determinants, such as dietary protein intake (related to vegetarianism), air and chemical pollution, and the high prevalence of infections in the community, has yet to be studied. The dietary protein intake (related to vegetarianism) influences disease progression (faster development of hypoalbuminemia and related complications and impact on the level of proteinuria), whereas prevalent infections have a role in disease development and course (IgAN, IC (Immune complex), and complement-mediated MPGN and postinfectious GN) and have an impact on risks with immunosuppressive therapies. In the Indian setting, it is also crucial to study the influence of access to care and develop and test cost-effective treatment approaches. The emerging precision therapeutic approaches are centered around biological agents, which are significantly more expensive. This makes it even more important to target their use to the population where the cost-effectiveness of their use is justified. There have been calls to test low-cost treatment strategies, including drug repurposing, where feasible. However, testing the newer or low-cost treatment therapies requires setting up of an efficient infrastructure. Randomized controlled trials (RCTs) are at the forefront of the evidence generation hierarchy and inform guideline recommendations. However, RCTs in nephrology, especially those involving patients with glomerular diseases, need to catch up to many internal medicine specialities. Unfortunately, even among the reported RCTs, representation from a limited-resource setting, especially among South Asians, is poor, despite them representing one-fifth of the world’s population.
To address the challenges mentioned above, we conceptualized the I-TANGIBLE network with the aim of developing a collaborative, integrated glomerular disease investigation infrastructure network for the following:
-
Carrying out clinical and translational studies in primary glomerular diseases
-
Identifying the risk factors and biomarkers of primary glomerular disease associated with poor short-term and long-term outcomes, including progressing to ESKD and death
-
Promoting new and improved strategies for diagnosis, individualized therapies, and prevention through collaborative research
-
Identifying factors determining adverse nonrenal outcomes like cardiovascular, bone health, and infections
-
Developing additional projects using the I-TANGIBLE database and biological sample storage framework
-
Setting up a clinical trial network for glomerular disease in alignment with global glomerular disease networks to conduct high-quality clinical trials.
Design and Methodology
Study design and objective
The I-TANGIBLE network consists of 13 participating institutions [Figure 1] in India, with George Institute for Global Health, New Delhi, India, being the coordinating center for the network, with responsibility for overall operational and quality control aspects of the study. At the end of the first year, we will contact other like-minded institutions in India to join the study network. A biorepository will be set up at the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh. Adults (>18 years) with biopsy-proven MCD, FSGS, MN, IgAN, or MPGN (immune complex- and complement-mediated), with a first biopsy taken within 2 years of enrollment, who provide informed consent will be included in the study. Patients with estimated glomerular filtration rate (eGFR) <15 ml/min/1.73 m2 for >3 months at the time of screening, kidney or bone-marrow transplant recipients, and patients with active malignancy or active hepatitis B/C replication or HIV-I/II will be excluded. This study will be conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines for Good Clinical Practice and the applicable local regulatory requirements for India. Ethical and governance approval has been obtained from The George Institute (TGI) India and from all network institutions.
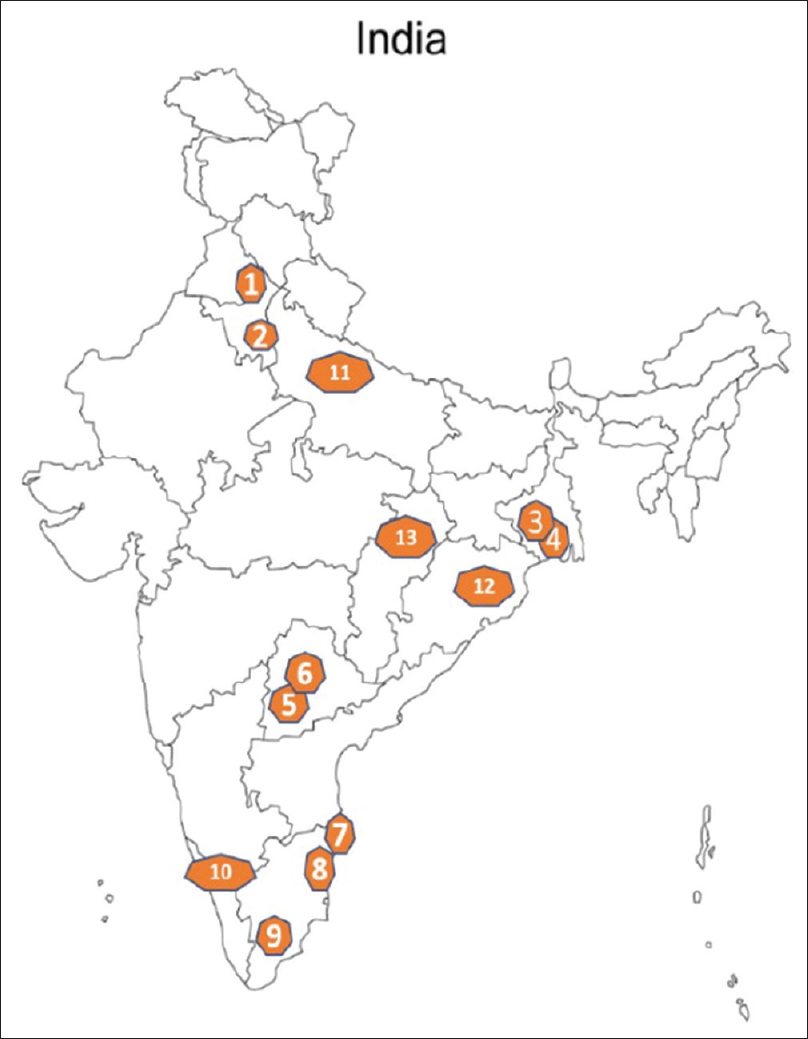
- Map of India showing location of study centers and geographic distribution: (1) Postgraduate Institute of Medical Education and Research, Chandigarh, (2) Safdarjung Hospital, New Delhi, (3) Institute of Postgraduate Medical Education and Research, Kolkata, (4) Nil Ratan Sircar Medical College, Kolkata, (5) Osmania Medical College, Hyderabad, (6) Nizam Institute of Medical Sciences, (7) Madras Medical College, Chennai, (8) Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, (9) Madurai Medical College, (10) Government Medical College, Calicut, (11) Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, (12) AIIMS Bhubaneshwar, (13) AIIMS, Raipur
Data collection and biobank
Participants will be recruited prospectively from the out-/in-patient lists of the participating centers. Study staff will screen renal biopsy lists, medical records, laboratory reports, and other existing site databases for participants who fit the study criteria. Participants will be approached to taken a written informed consent to provide their clinical data. Participants will also be approached for their willingness to contribute blood and urine samples for biorepository and for future research use including genetic research, linking their preliminary data and their willingness to be involved in any upcoming clinical trials by the site staff. Once written consent has been obtained, research staff will review the patients’ hospital record to gather their demographics, medical history, and glomerular disease diagnosis details. Data items to be collected at baseline and on follow-up are shown in Supplementary Material (Annexure I and II). The study participants will be managed by their treating teams as per the prevailing standard of care. Follow-ups will be synchronized with their routine follow-up [Supplementary Material, Annexure II] at 3 months, 6 months, 12 months, 18 months, 2 years and annually thereafter. All clinically relevant events will be recorded. We will store blood and urine samples at the time of diagnosis from patients who consent to this aspect of the study. Ten milliliters of blood samples will be collected at the sites using site-specific collection forms (specific to this project) and divided into aliquots. Samples will be processed and stored at −80°C.
| Annexure I: Baseline |
|---|
| Section A |
| 1. Patient ID |
| 2. Site ID |
| 3. Hospital record ID |
| 4. Has the patient provided consent for participating in the study? |
| 5. Date of consent |
| 6. Gender |
| 7. Age (completed years) |
| 8. First Name |
| 9. Last name |
| 10. Area |
| 11. City/village |
| 12. PIN |
| 13. Primary phone number (+1) |
| 14. Marital status |
| 15. Education |
| 16. Employment |
| 17. Modified Kuppuswamy score |
| 18. Health insurance status |
| 19. Family history of kidney disease |
| 20. Relevant past history |
| 21. CVD/hypertension/diabetes |
| 22. For women – obstetric history including h/o PE |
| 23. Functional status- HRQoL |
| Section B |
| 1. Duration of disease |
| 2. Presentation: nephrotic/nephritic/nephrotic–nephritic/RPRF/AUA |
| 3. Blood pressure: |
| 4. Total protein/serum albumin: |
| 5. Serum creatinine: |
| 6. Sodium/potassium |
| 7. 24-h urinary protein |
| Or |
| 8. Protein–creatinine ratio |
| 9. Urine microscopy: albumin−ve, trace, +, ++, +++, ++++; WBC: + (>5/HPF)/−; RBCs: + (>5/HPF)/−; glucose- +/− |
| 10. Serology: ANA/ANCA/anti-PLA2R/C3/C4 |
| 11. HBsAg, anti-HCV, HIV |
| 12. AST/ALT/TB/ALKP |
| Section C: Kidney biopsy |
| 1. Date |
| 2. Glomerular disease diagnosis: FSGS/MCD/MN/MPGN/IgA nephropathy |
| 3. Number of glomeruli |
| 4. Morphological description: Photo to upload or copy paste |
| Section D: Treatment |
| 1. Antihypertensive: CCB: yes/no, thiazide (or alike): yes/no |
| 2. Loop diuretic: yes/no |
| 3. ACEi/ARB: yes/no |
| 4. Steroids: no/yes, if yes, then (dose/duration) |
| 5. Cyclophosphamide: no/yes, if yes, then (dose/duration) |
| 6. CNI: No/Yes, if yes, then (dose/duration) |
| 7. Rituximab: no/yes, if yes, then (dose/duration) |
| 8. MMF: no/yes, if yes, then (dose/duration) |
| 9. Dialysis: no/yes |
| Annexure II: Follow-up |
| Date: |
| • Blood pressure (preferable home BP) |
| • Any new symptoms? |
| • If yes, provide details |
| • Functional status- HRQoL |
| • Serum creatinine |
| • 24-h urinary protein |
| • Serum albumin |
| • Urine microscopy |
| Treatment |
| 1. Antihypertensive: CCB: yes/no, thiazide (or alike): yes/no |
| 2. Loop diuretic: yes/no |
| 3. ACEi/ARB: yes/no |
| 4. Steroids: no/yes, if yes, then (dose/duration) |
| 5. Cyclophosphamide: no/yes, if yes, then (dose/duration) |
| 6. CNI: no/yes, if yes, then (dose/duration) |
| 7. Rituximab: no/yes, if yes, then (dose/duration) |
| 8. MMF: no/yes, if yes, then (dose/duration) |
| 9. Others: no/yes, if yes, then (dose/duration) |
| 10. Dialysis: no/yes |
| Complications (with details) |
| • AKI |
| • Thromboembolic |
| • Cardiovascular |
| • Infectious |
ACEi=angiotensin-converting enzyme inhibitors, AKI=acute kidney injury, ALKP=alkaline phosphatase, ALT=alanine aminotransferase, ANA=antinuclear antibodies, ANCA=anti-neutrophil cytoplasmic antibodies, ARBs=angiotensin receptor blockers, AST=aspartate aminotransferase, AUA=asymptomatic urinary abnormalities, BP=blood pressure, CCBs=calcium channel blockers, CNI=calcineurin inhibitor, CVD=cardiovascular disease, FSGS=focal segmental glomerulonephritis, HBsAg=hepatitis B surface antigen, HCV=hepatitis C virus, HIV=human immunodeficiency virus, HPF=high-power field, HRQoL=health-related quality of life, ID=identity, MCD=minimal change disease, MMF=mycophenolate mofetil, MN=membranous nephropathy, MPGN=membranoproliferative glomerulonephritis, PE=physical examination, PIN=postal index number, PLA2R=M-type phospholipase A2 receptor, RBCs=red blood cells, RPRF=rapidly progressive renal failure, TB=tuberculosis, WBCs=white blood cells
Study and data governance
A steering committee (SC) is already formed, whose members are the principal investigators (PIs) and key leaders in glomerular disease research in India. SC would be responsible for the general oversight of the study and provide scientific advice regarding all aspects of the study design, protocol development, conduct, and data collection. It will review the study progress and have a lead role in the study analysis and publication of results. Participant identifiers will be collected within the registry, and the data will be stored electronically in an electronic data capture system within the secure servers of TGI at New Delhi. All the sites will have access to their site-specific data. The ancillary studies’ applications will be scrutinized by the subcommittee of SC before submitting to the funding agencies.
Preliminary work
The study recruitment started in July 2022, and till 10 May 2023, we have recruited 745 participants: MCD (n = 190), FSGS (n = 206), MN (n = 302), and MPGN (n = 47).
Discussion
In the large, prospective I-TANGIBLE network, we will develop a well-characterized cohort of patients with common GN in South Asia that relates specific findings to a precise classification of glomerular diseases, risk stratification, development of biomarkers, treatment assignment and outcomes and create a suitable platform for evaluating newer or cost-effective therapies in clinical trials. Furthermore, integration of the clinical data set and subsequent translational research will enhance our understanding of pathogenesis and discover druggable molecular targets. The I-TANGIBLE network is the first initiative to bring together basic science and clinical and curative research in India and South Asia and will help localize global knowledge and develop predictive and management guidelines for our patients with GN.
This network will also facilitate the comparison of risk factors and outcomes with other international registries. Research capacity strengthening is a focus of the network. Owing to the rarity of certain glomerular diseases, limited access, and/or high costs of ancillary investigations like complement workup or modern immunosuppressive therapy,[27] combined with a lack of protocolized approach for managing glomerular diseases and long-term follow-up required for assessing clinically meaningful outcomes, there is an inadequate training of nephrology fellows in glomerular diseases. Given that the participating centers are institutions with nephrology training programs, we will generate a pool of well-trained nephrologists with interest in glomerular diseases. This is an important step in ensuring that the next generation of researchers and healthcare professionals are well equipped to tackle these diseases and develop new treatments.
Well-designed RCTs generate the most reliable evidence for testing newer therapies. Unfortunately, research suggests that both the quality and quantity of RCTs in nephrology need improvement, with particularly poor representation from limited-resource settings. If appropriately marshaled, the current clinical network will provide an apt platform for addressing these deficiencies. Increased participation in global clinical trials will increase the generalizability of clinical trial results and contribute to improved quality of care. In addition to these initiatives, I-TANGIBLE plans to develop clinical trials using modern approaches, such as platform trials. This could speed up the development of new treatments for glomerular diseases by allowing multiple treatments to be tested simultaneously and efficiently.
I-TANGIBLE is an aspirational network. The linked biobank will allow studies on gene expression profiling, genotyping, proteomics, and metabolomics to identify yet unknown biochemical and genetic biomarkers. Furthermore, it will be the first study to evaluate the prediction power of biologically important biomarkers on clinically relevant endpoints in the South Asian population. Such models are needed for predicting important outcomes and developing personalized therapies. The network plans to establish various programs and initiatives, including pilot and ancillary project programs, biomarker development using systems biology analysis, a digital image repository for scoring and using artificial intelligence (AI) algorithms, and collaborations with other glomerular disease networks for meta-analyses and international comparisons.
Challenges
Although the network is promising, we anticipate multiple challenges; they include loss of patients to follow-up, logistical challenges in collecting data in unprecedented situations like the coronavirus disease 2019 (COVID-19) pandemic, inability to recruit enough patients to the network, and loss of interest by the site investigators. Given that patients with GN often travel large distances to specialized centers for care, we expect dropouts because of patient movement. We will use an electronic retention system to collect essential data, including source data, remotely. During unprecedented situations like COVID-19 pandemic, we will try to engage the patients with telephone-based follow-up visits, with adjudication by site PI. Although slow recruitment can be a challenge, the preliminary work conducted during the last year suggests we should enroll a reasonable number of participants. Systematic long-term follow-up will help in assessing clinically relevant endpoints. Loss of interest among site investigators over time is a concern. To mitigate this risk, we will support the sites with study assistants, engage them through quarterly conferences, and encourage them to initiate and lead projects. Finally, sustainability is a challenge. The network has been set up with initial funding from the Indian Council of Medical Research (ICMR). We hope to both renew the funding and generate additional funding through clinical trials and additional discovery research grants that will support the network infrastructure. Though not foolproof, we believe our mitigation plans will address most of the concerns.
Strengths and limitations
This is the first formal registry of primary GN from a resource-limited setting, where the environment influencing the disease course may vary from the developed world. It will also provide data on health-related quality of life (HRQoL) assessment, a quintessential part of patient-reported outcomes. The proposed network aims to create a supportive framework that will serve as a basis for numerous clinical and translational studies in the future. Although the network does not directly answer fundamental questions, it will pave the way for research that can address important issues related to patient care and basic as well as translational research. Other limitations include the lack of protocolization of treatment, heterogenicity of recruited population, and a lack of representation from the pediatric population.
Conclusion
The I-TANGIBLE network will allow accurate ascertainment of disease burden of glomerular diseases in India, establish the treatment pattern of common glomerular diseases, and investigate medium- and long-term outcomes (remission, relapse, rate of eGFR decline).
Abbreviations
ID: identity, PIN: postal index number, CVD: cardiovascular disease, PE: physical examination, HRQoL: health-related quality of life, RPRF: rapidly progressive renal failure, AUA: asymptomatic urinary abnormalities, WBCs: white blood cells, HPF: high-power field, RBCs: red blood cells, ANA: antinuclear antibodies, ANCA: anti-neutrophil cytoplasmic antibodies, PLA2R: M-type phospholipase A2 receptor, HBsAg: hepatitis B surface antigen, HCV: hepatitis C virus, HIV: human immunodeficiency virus, AST: aspartate aminotransferase, ALT: alanine aminotransferase, TB: tuberculosis, ALKP: alkaline phosphatase, CCBs: calcium channel blockers, ACEi: angiotensin-converting enzyme inhibitors, ARBs: angiotensin receptor blockers, CNI: calcineurin inhibitor, MMF: mycophenolate mofetil, AKI: acute kidney injury.
Financial support and sponsorship
The project I-TANGIBLE is funded by ICMR, sanction number 5/4/7-1/Nephro/2021-NCD-II.
Conflicts of interest
Dr. Vivekanand Jha has received grant funding from GSK, Baxter, Astra Zeneca, Boerhringer Ingelheim, NephroPlus, and Zydus Cadilla, under the policy of all honoraria being paid to the organization. All the other authors do not have any conflict of interest to declare.
References
- What do we know about chronic kidney disease in India:First report of the Indian CKD registry. BMC Nephrol. 2012;13:10.
- [Google Scholar]
- Etiological profile of chronic kidney disease:A single-center retrospective hospital-based study. Saudi J Kidney Dis Transpl. 2018;29:409-13.
- [Google Scholar]
- Genetic regulation of serum IgA levels and susceptibility to common immune, infectious, kidney, and cardio-metabolic traits. Nat Commun. 2022;13:6859.
- [Google Scholar]
- Risk HLA-DQA1 and PLA (2) R1 alleles in idiopathic membranous nephropathy. N Engl J Med. 2011;364:616-26.
- [Google Scholar]
- Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol. 2016;27:3739-46.
- [Google Scholar]
- Traditional indigenous medicines are an etiologic consideration for NELL1-positive membranous nephropathy. Kidney Int. 2022;102:1424-6.
- [Google Scholar]
- Membranoproliferative glomerulonephritis--A new look at an old entity. N Engl J Med. 2012;366:1119-31.
- [Google Scholar]
- reverse phenotyping after whole-exome sequencing in steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2020;15:89-100.
- [Google Scholar]
- Selectivity and efficiency of utilization of galactosyl-oligosaccharides by bifidobacteria. Chem Pharm Bull (Tokyo). 1985;33:710-4.
- [Google Scholar]
- CureGN study rationale, design, and methods:Establishing a large prospective observational study of glomerular disease. Am J Kidney Dis. 2019;73:218-29.
- [Google Scholar]
- Design of the nephrotic syndrome study network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 2013;83:749-56.
- [Google Scholar]
- The Canadian Glomerulonephritis Registry (CGNR) and Translational Research Initiative:Rationale and Clinical Research Protocol. Can J Kidney Health Dis. 2022;9:20543581221089094.
- [Google Scholar]
- Information on rare kidney diseases. UK Kidney Association. Radar registry. Available from:https://ukkidney.org/rare-renal/radar
- [Google Scholar]
- Spectrum of steroid-resistant and congenital nephrotic syndrome in children:The PodoNet registry cohort. Clin J Am Soc Nephrol. 2015;10:592-600.
- [Google Scholar]
- PLA2R antibodies, glomerular PLA2R deposits and variations in PLA2R1 and HLA-DQA1 genes in primary membranous nephropathy in South Asians. Nephrol Dial Transplant. 2015;31:1486-93.
- [Google Scholar]
- Temporal association between PLA2R antibodies and clinical outcomes in primary membranous nephropathy. Kidney Int Rep. 2018;3:142-7.
- [Google Scholar]
- Complementary medicine and phospholipase A2 receptor (PLA2R)-related membranous nephropathy-fortuitous or causal? Kidney Int. 2023;103:425-7.
- [Google Scholar]
- Serial monitoring of anti-PLA2R in initial PLA2R-negative patients with primary membranous nephropathy. Kidney Int. 2015;88:1198-9.
- [Google Scholar]
- Antibodies to m-type phospholipase A2 receptor in children with idiopathic membranous nephropathy. Nephrology (Carlton). 2015;20:572-5.
- [Google Scholar]
- Utility of determining autoantibodies to M-type phospholipase A2 receptor in diagnosing primary membranous nephropathy:An ideal setting. Indian J Nephrol. 2017;27:413-5.
- [Google Scholar]
- Epidemiology, baseline characteristics and risk of progression in the first South-Asian prospective longitudinal observational IgA nephropathy cohort. Kidney Int Rep. 2021;6:414-28.
- [Google Scholar]
- Clinical and histopathologic profile of patients with primary IgA nephropathy seen in a tertiary hospital in India. Ren Fail. 2016;38:431-6.
- [Google Scholar]
- Primary IgA nephropathy in north India:Is it different? Postgrad Med J. 2012;88:15-20.
- [Google Scholar]
- Efficacy of corticosteroids in infection-related glomerulonephritis-a randomized controlled trial. Kidney Int Rep. 2022;7:2160-5.
- [Google Scholar]
- Outcome of C3 glomerulopathy patients:Largest single-centre experience from South Asia J Nephrol. . 2020;33:539-50.
- [Google Scholar]
- Challenges in diagnosis and management of glomerular disease in resource-limited settings. Kidney Int Rep. 2022;7:2141-9.
- [Google Scholar]







