Translate this page into:
Infection Patterns and Survival Among Renal Transplant Recipients
Corresponding author: Shrirang Bichu, Department of Nephrology, Bombay Hospital Institute of Medical Sciences, Mumbai, India. Email: shrirangbichu@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vishnu DS, Tilve P, Bodke SY, Deb S, Andankar M, Oza U, et al. Infection Patterns and Survival Among Renal Transplant Recipients. Indian J Nephrol. doi: 10.25259/ijn_453_23
Abstract
Background
The outcome of kidney transplantation is determined by multiple factors and infections represent one of the major factors affecting graft and patient survival. Recent COVID-19 pandemic have adversely affected the transplant population. Very little data is available on post-transplant infections and patient survival from India.
Materials and Methods
In this retrospective observational study, data related to post-transplant infections from patients who had undergone renal transplantation between October 2014 and October 2021 were collected.
Results
A total of 255 infections episodes were observed in 118 patients. Bacterial infections were the most common (55%) followed by viral (35%), fungal (5%), mycobacterial (4%), and parasitic (1%). The most common bacterial and viral infections were urinary tract infections (70.5%) and COVID-19 (56%), respectively. BK virus and COVID-19 were associated with increased graft loss (p < 0.05). The majority of deaths due to infections were related to COVID-19 infection (71.42%). Kaplan-Meier survival analysis showed 1-, 3-, and 5-year patient survival of 98.23%, 96.36%, and 92.90% and graft survival of 98.14%, 95.97%, and 91.78, respectively.
Conclusion
Infections with their adverse impact remain a concern in kidney transplant patients. Comparable patient and graft survival to the Western data despite the high infection burden and the COVID-19 pandemic suggests that effective management can reduce the impact of infections on survival.
Keywords
Kidney transplantation
Infections
Survival
Introduction
Infections are an important cause of mortality and morbidity, with roughly one-quarter of renal transplant recipients experiencing serious infections that lead to allograft dysfunction.1 In tropical countries, the incidence of post-transplant infections ranges from 50% to 70%,2 demonstrating a distinct pattern compared to developed regions.3 Factors such as poor sanitary conditions, climate changes in the tropics, late presentation, illiteracy, lack of knowledge, poor diagnostic techniques, and poor availability of lifesaving medicines had contributed to poor post-transplant outcomes in some developing countries.1 In contrast to the developing world where cardiovascular mortality predominates, India sees infections as a leading cause of death.4,5 The initial 6 months post-transplant present a critical period characterized by a peak cumulative dose of immunosuppression, thereby increasing the incidence and spectrum of infections during this timeframe.6 The recent COVID-19 pandemic further impacted transplant recipients.7 This study aims to comprehensively assess the infection patterns in post-kidney transplant patients. The study endeavors to explore the impact of these infections of both patient and graft survival rates.
Materials and Methods
This retrospective observational study was conducted by a single nephrology unit, focusing on 118 patients who underwent renal transplantation between October 2014 and October 2021. Being a retrospective study, waiver of consent was obtained from ethics committee of Bombay Hospital. Patients with <1 year follow-up at the time of study recruitment were excluded. We included patients exclusively from a single unit to maintain consistency in management protocol. All patients who have completed 1 year after renal transplantation were included. There were no other exclusion criteria. Relevant data included demographic details, patient characteristics (comorbidities, native kidney disease, etc), transplant details (induction agent, ABO compatibility, type of donor, double J stent use, immunosuppression regimen, etc), and post-transplant course (graft function, infections, rejection episodes, allograft biopsies, post-transplant diabetes mellitus – PTDM, etc).
For induction, we use anti thymocyte globulin (ATG) 3 to 4.5 mg/kg in all except those with 2 haplotype match donor. We use tacrolimus, mycophenolate mofetil and prednisolone for maintenance immunosuppression. We start mycophenolate mofetil 1 gm twice a day and tacrolimus 0.1 mg/kg in two divided doses, 5 and 2 days prior to surgery respectively. Intravenous methylprednisolone 1 gm, 500 mg, 375 mg and 250 mg are administered on days 0, 1, 2 and 3 of the operative period followed by oral prednisolone 1 mg per kg per day which is tapered to 20 mg per day by the time of discharge on day 8-10 of the post operative period. Prednisolone is further tapered over the next few weeks to a maintenance dose of 5 mg per day by 3 months.
We targeted trough levels of tacrolimus of 8 to 9 ng/mL [chemiluminiscence immunoassay (CLIA) method] immediately post-transplant, then 7–8 ng/mL for 1 month, thereafter 6–7 ng/mL for 3 months till 1 year, and 4–6 ng/mL after 1 year. We used Valganciclovir in all patients who have received an induction agent for 100 days.
Timethoprim-sulfamethoxazole prophylaxis was used for 6 months and antifungal prophylaxis in the form of clotrimazole mouth paint and antiseptic gargles for 6 months.
In the case of patients with chronic diarrhea, we have reviewed dietary habits and changed non-immunosuppressive medications. Then, the search for infective causes was done. In the case of patients who have gastrointestinal intolerance due to mycophenolate mofetil, we shifted to mycophenolate sodium formulation. If still persistent, we split the dose to three times a day and subsequently reduced the dose by 50%. If the patient does not respond to this, we shifted to azathioprine after doing a thiopurine methyl transferase (TPMT) test to check enzyme plasma levels.
In the case of CMV, the antimetabolite and targeted tacrolimus trough levels were kept between 4 and 5 ng/mL (CLIA method).
In the case of BK viremia, mycophenolate mofetil was reduced by 50%, and tacrolimus continued at reduced dose and observed for the response. Further reduction in immunosuppression was done according to BKV PCR results.
Patients were followed up twice a week for 6 weeks followed by once a week for 6 weeks, then monthly for 1 year, and 3 monthly thereafter.
The outcomes were the number of infections episodes, type of infection and pathogen, timing and site of infection, and patient and graft survival. Graft dysfunction was defined as an increase in serum creatinine of 15% from baseline,8 which included both acute and chronic graft dysfunction and graft failure as a return to dialysis.9 At the time of enrolment, acute graft dysfunction (graft dysfunction <3 months) was present in six patients (14.28%) and chronic allograft dysfunction (graft dysfunction >3 months) in 36 (85.71%). Induction therapy was given to all, but three patients and the maintenance regimen consisted of tacrolimus, mycophenolate mofetil, and prednisolone. Institutional ethics committee approval was obtained, and the standard protocol was followed.
Statistical analysis
Quantitative variables were summarized using mean and standard deviation (SD). Categorical variables were represented using frequency and percentage. Chi-square test and Fisher’s exact tests were used to assess associations between risk factors and infections, with a significance level of p<0.05. Survival analysis was done by Kaplan-Meier survival analysis curves. We have censored death as a cause of graft loss in our study.
Results
Demographic data are summarized in Table 1. The majority were in the 30–50 age group (53%), while 15% were >60 years old. Median follow-up was 43 months (interquartile range 28–64 months). Biopsy-proven acute graft rejections were seen on 34 biopsies (ACR – 23, ABMR – 6, combined ACR and ABMR – 5) and chronic rejections were seen in six patients.
| Characteristics of patients | Value* |
|---|---|
| Age of recipients (years), mean ± SD | 44.54 ± 13.03 |
| Gender of recipients (female:male) | 35 (29.66):83 (70.34) |
| Comorbidities | |
| DM | 30 (25.42) |
| HTN | 84 (71.18) |
| IHD | 15 (12.71) |
| Preemptive transplant | 14 (11.86) |
| ABOi transplant | 3 (2.54) |
| Type of transplant | |
| Cadaver | 21 (17.79) |
| Living | 97 (82.20) |
| Induction agent used | |
| ATG | 113 (95.76) |
| Basiliximab | 02 (01.92) |
| None | 03 (2.54) |
| DJ stent used | 110 (93.22) |
| Mean duration of DJ stent | 23.25 + 9.3 days |
| Tuberculosis exposure Pre-Transplant | 9 patients |
| Pediatric transplants | 1 patient |
| Cause of ESRD | DM 27, HTN 21, CGN 12, unknown 23, ADPKD 11, VUR 3, CTID 5, IgAN 9, vasculitis 2, CAKUT 2, obstructive causes 3 |
| PTDM | 20 patients |
| % of underlying lung disease in comorbidities | 9 patients had h/o tuberculosis prior to transplant, 1 patient had OSA, and 2 patients had COPD |
| Mean creatinine or eGFR at discharge | 1.18 |
| % with chronic allograft injury | 36 patients |
| % received antirejection therapy | 34 |
No patient was lost to follow-up. A total of 255 infections episodes among the 118 transplant recipients were noted throughout the study period. 36 patients had three or more infections episodes and nine patients had five or more infections episodes. Figures 1-3 show the distribution of infections episodes according to timeline, causative pathogens, and site of infection, respectively. Table 2 represents the timeline of infections according to post-transplant duration. Bacterial infections were the most common (55%) followed by viral (35%) and fungal (5%). Urinary tract infection (UTI) was the predominant bacterial infection, occurring in 55 (70.5%) patients followed by bacterial respiratory infection in 23 (29.5%) cases. Tuberculosis affected 11 (4%) patients. Out of this pulmonary involvement was seen in three, lymph node in five, graft in one, abdominal in one, and disseminated involvement in one case. Among viral infections, COVID-19 infection (56%), CMV (18%), and herpes infection (17%) were the most frequent. We found patients with CMV had more infections (p = 0.03) highlighting the immunomodulatory role of CMV. Incidence of infection risk positively correlated with anti-rejection therapy, with Odd’s ratio of 2.0 (p = 0.06).
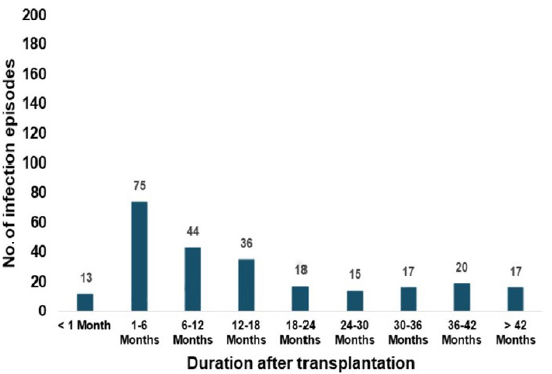
- Distribution of post-transplant infection episodes according to the timeline.
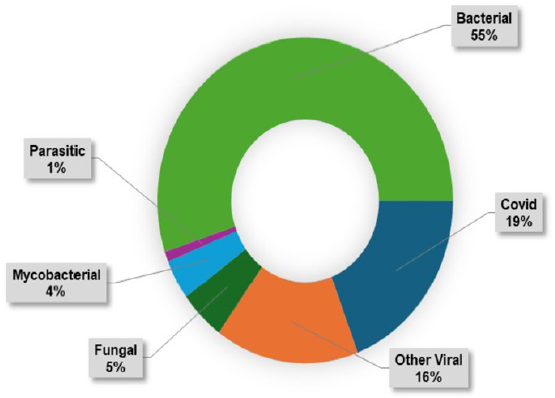
- Distribution of post-transplant infection episodes according to etiology.
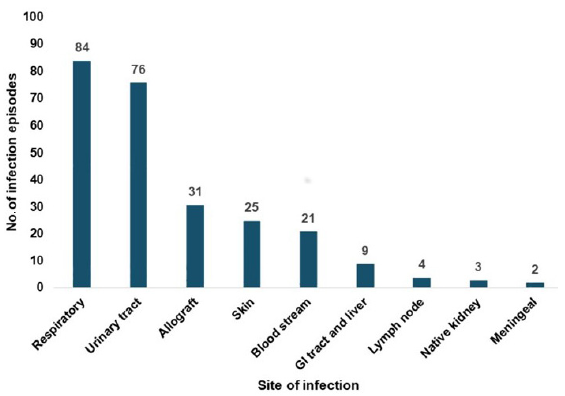
- Distribution of post-transplant infection episodes according to the site of infection. GI tract- Gastrointestinal tract
| Type of infection | Timeline of infection | |||
|---|---|---|---|---|
| <1 month | 1–6 months | 6–12 months | >12 months | |
| CMV | 0 | 4 | 7 | 5 |
| BK | 0 | 0 | 3 | 2 |
| TB | 0 | 1 | 3 | 7 |
| UTI | 9 | 14 | 12 | 22 |
CMV: Cytomegalovirus, TB: Tuberculosis, UTI: Urinary tract infection
Graft dysfunction was reported in 42 (36%) patients. Nine patients experienced graft failure. Table 3 shows an analysis of factors associated with overall patient mortality. Multivariate analysis for patient mortality showed an association with age, DM, and graft dysfunction (p <0.05). Risk factors analyzed were age, gender, DM, deceased donor, graft dysfunction, induction agent, h/o infections like UTI, CMV, BKV, TB, and COVID-19. Multivariate analysis for graft loss showed an association with DM (p-value 0.03), graft dysfunction (p-value 0.001), BKV (p-value 0.023), and COVID-19 (p-value 0.045). Risk factors analyzed were age, gender, DM, deceased donor, induction agent, number of UTI episodes, history of CMV, history of BKV, history of tuberculosis, and history of COVID-19.
| Factors associated with mortality | Odd’s ratio (OR) | 95% Confidence interval | p-value |
|---|---|---|---|
| Age > 60 years | 5.885 | 1.465–23.11 | 0.006 |
| DM | 10.03 | 2.527–50.14 | <0.001 |
| Deceased donor | 2.989 | 0.698–11.52 | 0.065 |
| CMV | 1.471 | 0.198–7.002 | 0.314 |
| BKV | 2.546 | 0.094–22.67 | 0.230 |
| UTI | 1.605 | 0.385–5.976 | 0.241 |
| COVID-19 | 1.191 | 0.316–4.333 | 0.78 |
| Biopsy-proven acute graft rejections | 1.863 | 0.445–6.978 | 0.182 |
| Any history of graft dysfunction (acute/chronic) | 9.88 | 2.211–70.32 | <0.001 |
| Graft loss | 1.235 | 0.0505–8.96 | 0.398 |
DM: Diabetes Mellitus, CMV: Cytomegalovirus, BKV: BK virus, UTI: Urinary tract infection
Overall mortality seen in our study was 11 (9%) of which infection-related mortality was seen in seven cases. Kaplan-Meier analysis [Figure 4] showed 1-, 3-, and 5-year graft survival of 98.14%, 95.97%, and 91.78%, respectively. Kaplan-Meier survival analysis [Figure 5] showed 1 year, 3 years, and 5 years patient survival of 98.23%, 96.36%, and 92.90%, respectively. In our study, nine grafts were lost and out of the 11 deaths, 10 died with functioning grafts. Table 4 shows survival rates according to frequency of infections in post-transplant period. Figure 6 shows patients’ survival graph based on number of infections.
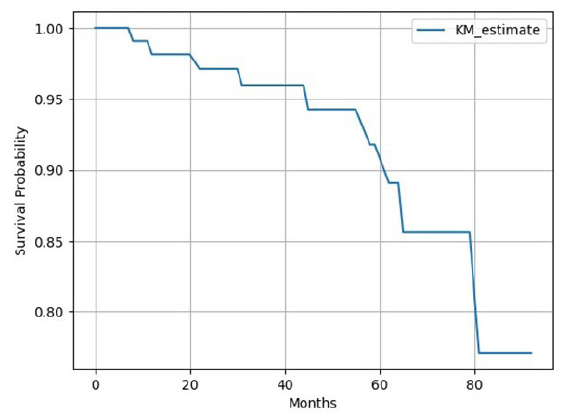
- Kaplan-Meier (KM) survival analysis for graft survival.
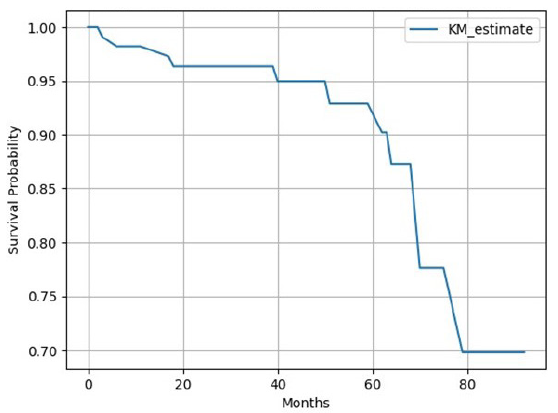
- Kaplan-Meier (KM) survival analysis for patients’ survival.
| Year after renal transplantation | Survival rate in patients with 2 or less than 2 infection episodes | Survival rate in patients with more than 2 infection episodes |
|---|---|---|
| 1 | 98 | 96 |
| 2 | 97 | 92 |
| 3 | 97 | 92 |
| 4 | 97 | 88 |
| 5 | 93 | 88 |
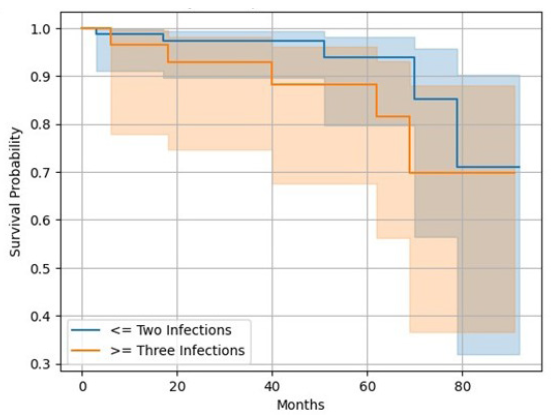
- Kaplan-Meier survival analysis for patients’ survival based on the number of infections.
Discussion
The study focuses on post-transplant infections in western India, highlighting their significant impact on mortality and morbidity. In contrast to Western countries where cardiovascular disease4 is the leading cause of death among transplant recipients, India sees infections as the primary cause of mortality.5 Our center experienced a drop in the annual kidney transplantation numbers due to the COVID-19 pandemic, similar to the observations across centers in India.7 The 100% follow-up in our study population can be attributed to thorough counseling sessions held on multiple occasions with patients and their immediate family members. The primary focus was to highlight the importance of adhering to a rigorous follow-up routine for optimal graft and patient outcomes. Bacterial infections were the most common, followed by viral and fungal. These results are in accordance with a study by Mahapatra et al.10 Most infections (65%) occurred more than 6 months after transplantation, suggesting community-acquired or opportunistic infections.
Urinary tract infections were the most common bacterial infections in 55 (70.5%) patients. Gupta et al. also reported UTI as the most common bacterial infection, followed by pneumonia.11 Forty-four patients suffered from Pyelonephritis, and 11 had cystitis. We use Amoxicillin-clavulanic acid, levofloxacin, trimethoprim-sulfamethoxazole, and Fosfomycin in cystitis. For graft pyelonephritis, the protocol is to admit the patient and start empirical IV antibiotics, for example, IV Cefoperazone-sulbactam, piperacillin-tazobactam, or IV meropenem and then change to sensitive antibiotics according to culture reports. Antibiotics are continued for a total duration of 2–3 weeks. In the protocol of prophylaxis after UTI episodes, we consider secondary prophylaxis for 6 weeks with oral antibiotics depending on the sensitivity report, in recurrent UTI cases.
Bacterial respiratory infections were seen in 23 (29.5%) patients. This finding is comparable to an Indian study by Mangalgi et al., which reported bacterial respiratory infections in 29.4% of patients.12
In our study, 32 patients suffered at least one episode of UTI, and 13 patients had suffered >3 UTI episodes (24%). The most common organisms were Escherichia coli (32%), Klebsiella (16%), and Pseudomonas (16%). Other studies also reported E. coli as a commonest organism in post-transplant UTI.10,13 Nine patients had bladder dysfunction. Risk factors for UTI analysis showed female gender (0.04), DM (0.05), history of prior UTIs (0.04), anti-rejection therapy (0.03) are significantly associated with post-transplant UTI risk (p<0.05). We also evaluated risk factors like age, gender, DM, deceased donor, induction agent use, pre-transplant history of UTI, history of CMV, history of BKV, history of TB, history of COVID, antirejection therapy, structural urinary abnormalities, and presence of DJ stent.
Tuberculosis was reported in 9.3% cases. Indian studies show a higher prevalence of post-transplant tuberculosis ranging between 8% and 13%.14–16 Our results are comparable with these studies. Nocardia infection was seen in four (3%) patients. Among them, three had pulmonary involvement and one disseminated involvement with no case fatality noted. One Indian study reported a 1.4% incidence of Nocardia17 in solid organ transplants. Other studies have reported incidence varying from 0.7% to 3.5%.18
COVID-19 was the most common viral infection in the study. Forty-nine patients had COVID-19, and the COVID mortality rate was 10.2%. Kute et al.19 reported an overall patient mortality of 11.6%. Out of 49 COVID patients, 21 patients developed graft dysfunction post-COVID-19 period, and we found the association between COVID, and post-COVID graft dysfunction was not significant (p-value of 0.2 using Chi-square test, OR 0.6).
CMV infection was seen in 16 (14%) patients. Kumar et al. reported a 13.3% prevalence of CMV20 whereas John et al. reported a prevalence of 20%.20,3 Herpes virus is the third most common viral infection seen. Herpes simplex infection occurred in 12 (10%) patients – an incidence similar to that in the study by Jha et al. (11%).2 Varicella zoster (chicken pox) infection occurred in three patients. BK virus infection was seen in five (4%) patients; with one patient losing the graft to BK nephropathy. In a study by Dadhania et al.21 and Hirsch et al.,22 there was a prevalence of 10% of BKV nephropathy in kidney transplant recipients.
Fungal infections were seen in 13 (11%) patients, including one case of pulmonary aspergillosis and one case of cryptococcal meningitis. Other centers from India have reported incidences ranging from 2.5% to 19%.23 Singh et al. have reported invasive aspergillosis in 0.7%–4% of renal transplant recipients.24 PCP pneumonia was seen in one patient. Rodriguez et al., reported the incidence of PCP in renal transplant recipients as 0.6%–14%.25
Overall mortality was 9%, with infection contributing to seven out of the 11 deaths. COVID-19 was the most common infective cause leading to mortality in five out of the seven infection-related deaths. Five patients who died due to COVID-19 were not vaccinated prior to infection. Out of the two cases of non-COVID-related mortality, one had lower respiratory tract infection due to pseudomonas with multiorgan involvement, and one had pyelonephritis sepsis with septic shock. A study by Mahapatra et al.10 reported 39.1% of graft losses during the COVID-19 pandemic. Age >60 years, history of DM, and graft dysfunction were significantly associated with patient mortality (p<0.05). Other factors increasing the likelihood of deaths in transplant recipients were deceased donor transplants, biopsy-proven graft rejections, infections like CMV, BKV, COVID-19, UTI, and graft failure (with OR > 1). Nine patients had graft failure and among infections, COVID-19 and BK virus were significantly associated with graft loss (p < 0.05).
Graft survival rates at 1, 3, and 5 years were 98.14%, 95.97%, and 91.78%, respectively [Figure 4]. Jha et al. have reported death-censored one-year graft survival of 97.6% during the COVID pandemic period.7 In another Indian study, death-censored renal allograft survival at 1, 3, and 5 years were 94%, 90%, and 79%, respectively.26 Data from the National Scientific Registry of Transplant Recipients (SRTR) from the US reported that in living donor transplants, graft survival at 1 and 5 years was 97.8% and 86.5%, respectively.27 Also, a study by Sundaram Hariharan in 2021 reported a 5-year graft survival rate in the US (85%), Europe (87%), Australia and New Zealand (90%), and Canada (91%).28 Death with graft function (DWGF) as a percentage of total mortality seen in our study was 90.9%. Indian study by Prakash et al. reported a DWGF rate in 88% of patients.5
Patient survival rates at 1, 3, and 5 years were 98.23%, 96.36%, and 92.90%, respectively [Figure 5]. One Indian study showed patient survival rates of 92%, 87%, and 83% at 1, 3, and 5 years, respectively.26 Patient survival with graft function at 1, 5, and 10 years was reported as 97%, 91%, and 86%,29 by a study done in the United States. Survival analysis showed that patients with <= 2 infections had more survival benefits than >2 infections (93 years vs. 88 years). Figure 6 shows survival analysis based on infection episodes. In our study, patient and graft survival rates are comparable with existing Indian as well as international data although the incidence of infection is high (100%) despite the COVID-19 pandemic during the study period.
The novel findings in our study include, in spite of having higher infection rates, we observed overall graft and patient survival, comparable to international standards and this emphasizes the importance of timely diagnosis and appropriate treatment. Diabetes mellitus continues to be a major association with overall infection risk as well as graft and patient survival; thus, highlighting diabetes as an important risk factor for transplant outcomes. Our study includes the duration of the COVID-19 pandemic, highlighting its influence on survival and graft outcomes among patients affected by COVID-19.
Some limitions would be that this was a single-unit, retrospective study with a relatively modest sample size. Comprehensive data on pre-transplant vaccination status and tissue typing were not readily accessible.
Conclusion
Infections with their adverse impact on graft and patient survival remain one of the major concerns in kidney transplant patients. Prompt diagnosis and treatment of infections, combined with tight titration of immunosuppressive medications, continues to be crucial in addressing the high burden of infections in the tropics. Our study has also contributed to understanding the impact of the COVID-19 pandemic on survival and graft outcomes in our unit. The patient and graft survival in this study was comparable to the Western data suggesting that effective management can reduce the impact of infections on patient and graft survival.
Acknowledgement
Dr. Vaishali Thakare, Associate Professor, Department of Pharmacology, D Y Patil Medical College, Navi-Mumbai.
Conflicts of interest
There are no conflicts of interest.
References
- Time table of infections after renal transplantation – South Indian experience. Indian J Nephrol. 2005;15:S14-S21.
- [Google Scholar]
- Infections in dialysis and transplant patients in tropical countries. Kidney International. 2000;57:S85-S93. 10.1046/j.1523-1755.2000.07415.x
- [Google Scholar]
- Rates of first infection following kidney transplant in the United States. Kidney Int. 2009;75:317-26.
- [CrossRef] [PubMed] [Google Scholar]
- Causes of death in renal transplant recipients with functioning allograft. Indian J Nephrol. 2012;22(4):264-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Danovitch GM, ed. Handbook of kidney transplantation (6th ed). Wolters Kulwer; 2017.
- A retrospective multi-center experience of renal transplants from India during COVID-19 pandemic. Clin Transplant. 2021;35:e14423.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Post-transplantation Monitoring and Outcomes. In: Primer on Kidney Diseases. Elsevier; 2009. p. :534-542.
- [CrossRef] [Google Scholar]
- Coming back to dialysis after kidney transplant failure. Nephrol Dial Transplant. 2008;23:2738-42.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum of infections in renal transplant recipients, factors affecting long term patient and graft outcomes over 10 years including COVID pandemic periods. Indian J Transplant. 2023;17:16.
- [Google Scholar]
- Opportunistic infections occurring in renal transplant recipients in tropical countries. Indian J Transplant. 2019;13:110.
- [Google Scholar]
- Pulmonary infections after renal transplantation: A prospective study from a tropical country. Transplant Int. 2021;34:525-34.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary tract infections in the era of newer immunosuppressant agents: A tertiary care center study. Saudi J Kidney Dis Transpl. 2010;21:876-80.
- [PubMed] [Google Scholar]
- Tuberculosis in renal transplant recipients: Our decade long experience with an opportunistic invader. Indian J Tuberc. 2020;67:73-8.
- [CrossRef] [PubMed] [Google Scholar]
- A retrospective study to assess dropout rate and its causes in living donor transplant program in India. Kidney International Reports. 2022;7:S348.
- [Google Scholar]
- Impact of type of calcineurin inhibitor on post-transplant tuberculosis: Single-center study from India. Transpl Infect Dis. 2017;19
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors, clinical characteristics, and outcome of nocardia infection in organ transplant recipients: A matched case-control study. Clin Infect Dis. 2007;44:1307-14.
- [CrossRef] [PubMed] [Google Scholar]
- Disseminated nocardiosis in kidney transplant recipients: A report of 2 cases. Kidney Med. 2022;4:100551.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical profile and outcome of COVID-19 in 250 kidney transplant recipients: A multicenter cohort study from India. Transplantation. 2021;105:851-60.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Profile of infections in renal transplant recipients from India. J Family Med Prim Care. 2016;5:611-14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology of BK virus in renal allograft recipients: Independent risk factors for BK virus replication. Transplantation. 2008;86:521-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13:179-88.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of systemic mycoses among renal-transplant recipients in India. Transplantation. 2003;75:1544-51.
- [CrossRef] [PubMed] [Google Scholar]
- Aspergillosis in solid organ transplantation. Am J Transplant. 2013;13:228-41.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus-negative immunocompromised patients. Clin Microbiol Rev. 2004;17:770-82.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Predictors of allograft survival and patient survival in living donor renal transplant recipients. Indian J Transplant. 2017;11:42.
- [Google Scholar]
- Long-term kidney transplant graft survival—Making progress when most needed. Am J Transplant. 2021;21:2824-32.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term survival after kidney transplantation. N Engl J Med. 2021;385:729-43.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307-13.
- [CrossRef] [PubMed] [Google Scholar]







