Translate this page into:
Insights into Therapeutic Strategies and Longitudinal Outcomes: A Retrospective Analysis of NELL1 Positive Membranous Nephropathy Cohort
Corresponding author: Gurjot Singh, Department of Nephrology, Sawai Man Singh Medical College Jaipur, India. E-mail: iisham.gs@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh G, Makhija H, Beniwal P, Malhotra V. Insights into Therapeutic Strategies and Longitudinal Outcomes: A Retrospective Analysis of NELL1 Positive Membranous Nephropathy Cohort. Indian J Nephrol. 2025;35:87-90. doi: 10.25259/IJN_443_2024
Abstract
Membranous nephropathy (MN) is a rare autoimmune disease, in which the circulating autoantibodies against antigens attack podocytes. Neural Epidermal Growth Factor like 1 (NELL1) 1-associated MN is the second most common antigen, following phospholipase A2 receptor. Complementary and alternative medicine and malignancies play a pivotal role in the development of NELL1-MN. This retrospective study describes the clinical characteristics, therapeutic strategies, and longitudinal outcomes in patients with NELL1-MN at our center.
Keywords
Membranous nephropathy
NELL1
PLA2R
Complementary and alternative medicine
CNI
Introduction
Membranous nephropathy (MN) is a rare autoimmune disease, in which circulating autoantibodies against antigens attack podocytes. It accounts for about 20% cases of nephrotic syndrome in adults.1 Sethi et al. using laser microdissection and tandem mass spectrometry (MS/MS) have recently described six new target antigens in MN that include neural tissue encoding protein with EGF-like repeats [Neural Epidermal Growth Factor like 1 (NELL1)] and others like Exostosin-1, Semaphorin3B.2 NELL1 is the second most common antigen implicated in development of MN.3 Emerging evidence suggests that complementary and alternative medicine (CAM) and malignancies may play a pivotal role in the development of NELL1-associated MN (NELL-1 MN).4-6 Many cases of NELL-MN remit following stoppage of the offending agent but some may require immunosuppression. We examined the clinical characteristics, therapeutic strategies, and longitudinal outcomes of NELL1-MN patients at our center.
Case Series
All adult cases with NELL1-MN diagnosed in Nephrology Department of Sawai Man Singh Medical College & Hospital, Jaipur, India between April 1, 2021 and March 31, 2023 were identified from hospital records and contacted via telephone. Data on demographic parameters, biochemical, and histological characteristics were collected. Immunosuppression and treatment history along with baseline and last available serum albumin, creatinine, and proteinuria were recorded. Those with CKD stage 3 or above at presentation, patients with lupus nephritis or other connective tissue disorders were excluded.
Of the 15 patients with NELL1-MN identified, data of 10 patients were available for analysis as depicted in Table 1. There were 9 females and the mean age was 37.2±15.2 years. The mean duration of follow-up since biopsy was 18.2±6.9 months.
| Patient | Baseline parameters (at presentation) | Associated features | Renal biopsy findings | Follow-up (months) | ACEi/ARB | 24-hr UP after 3–6 mo (mg/d) | Immunosuppression (months) | Latest parameters | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
S. Cr (mg/dL) |
24-hr UP (mg/d) |
S. Alb (g/dL) |
S. Cr | 24-hr UP | S. Alb | ||||||||
| 43/M | 1.3 | - | 2.2 | None | IFTA 20-25%, IgG 3+, C3 2+ | Excluded (Lost to follow-up) | - | - | - | - | - | - | - |
| 68/M | 0.9 | 2800 | 2.8 | Psoriais, HTN, CAM | IFTA 10-15% IgG1, IgG4 3+ granular along capillary wall,C3 1+ | 33 | Yes (33 mo) | 1300 | No | 0.8 | 281 | 3.8 | CR |
| 27/F | 0.7 | 12,000 | 1.8 | None | IFTA 10-15%IgG1, IgG4 3+ segmental & capillary wall, granular,C3 1+ | 27 | Yes (27 mo) | 10070 | CNI + P (12 mo) | 1.3 | 211 | 3.5 | CR |
| 42/F | 0.9 | 2190 | 2.8 | None | IFTA 10-15%IgG1 3+, IgG4 3+ capillary wall, granular,C3 -ve | 24 | Yes (24 mo) | 1584 | No | 0.5 | 127 | 3.8 | CR |
| 31/F | 0.82 | 14,767 | 2.6 | CAM intake | IFTA 10-15%IgG1 3+, IgG4 1+ capillary wall, granular,C3 1+ | 19 | Yes (12 mo) | 12600 | CNI + P (6 mo) Modified Ponticelli (6 mo) | 2.9 | 2190 | 2.4 | GFR decline |
| 32/F | 0.65 | 8800 | 2.87 | Migraine, NSAID intake | IFTA 10-15%IgG1 3+, IgG4 3+ capillary wall, granular,C3 -ve | 15 | Yes (15 mo) | 9800 | CNI + P (12 mo) | 0.7 | 420 | 3.9 | PR |
| 22/M | 1.25 | 2130 | 2.6 | None | IFTA 10-15% IgG1 3+, IgG4 1+ capillary wall, granular,C3 2+ | 14 | Yes (14 mo) | 354 | No | 0.9 | 82 | 4.1 | CR |
| 64/M | 0.94 | 4490 | 2.6 | HTN, Osteoarthritis, NSAID use | IFTA 10-15% IgG1 3+, IgG4 2+ capillary wall, granular,C3 1+ | 13 | Yes (13 mo) | 5420 | CNI + P (10 mo) | 0.6 | 291 | 3.8 | CR |
| 28/F | 0.6 | 7120 | 2.3 | Chronic Headache, NSAID intake | IFTA 10-15% IgG1 3+, IgG4 3+ capillary wall, granular,C3 1+ | 13 | Yes (13 mo) | 6800 | CNI + P (10 mo) | 0.8 | 380 | 4 | PR |
| 36/M | 5.4 | - | 2.2 | CKD,HTN | Glom- 38% globally sclerosed, IFTA 35-40% IgG 2+, capillary wall, granular,C3 1+ | Excluded (eGFR <60) | - | - | - | - | - | - | - |
| 30/M | 0.8 | 7640 | 2.1 | None | IFTA 10-15% IgG1, IgG4 3+ segmental & capillary wall, granular,C3 1+ | 12 | Yes (12 mo) | 10880 | CNI + P (9 mo) | 0.7 | 795 | 3.6 | PR |
| 28/F | 0.75 | 3090 | 2.72 | Atopy, Old Pulmonary TB, CAM | IFTA 10-15% IgG1, IgG4 3+ segmental & capillary wall, granular,C3 -ve | 12 | Yes (12 mo) | 3800 | CNI + P (6 mo) | 1.1 | 660 | 3.4 | PR |
| 34/F | 0.8 | - | 2.4 | Multiple food allergies | IFTA 10-15% IgG 3+ capillary wall, granular, C3 2+ | Excluded (<1 year follow up) | - | - | - | - | - | - | - |
| 57/F | 0.9 | - | 2.6 | None | IFTA 10-15% IgG 3+ capillary wall, granular,C3 3+ | Excluded (<1 year follow up) | - | - | - | - | - | - | - |
| 30/F | 1 | - | 2 | Rash, alopecia | IFTA 10-15% IgG 3+ capillary wall, granular,C3 2+ | Excluded (<1 year follow up) | - | - | - | - | - | - | - |
ACEi: Angiotensin converting enzyme inhibitor, Alb: Albumin, ARB: Angiotensin receptor blockers, CAM: Complementary and Alternative Medicine, CKD: Chronic Kidney Disease, CNI: Calcineurin inhibitor, CR: Complete remission, Cr: Creatinine, C3: Complement factor 3, eGFR: estimated Glomerular Filtration Rate, HTN: Hypertension, IFTA: Interstitial fibrosis and tubular atrophy, IgG: Immunoglobulin G, NSAID: Non-Steroidal Anti-Inflammatory Drug, P: Prednisolone, PR: Partial remission, TB: Tuberculosis, UP: Urine Protein
Three patients each had a history of CAM and NSAID intake. None had any history of skin fairness cream use, any identifiable malignancy or autoimmune disease. One patient had history of psoriasis, another of atopy, and two with migraine and another two of hypertension. On histopathology, all patients had IgG1 and IgG4 positivity. C3 positivity was seen in 70% and segmental staining in 30% of cases [Figure 1].
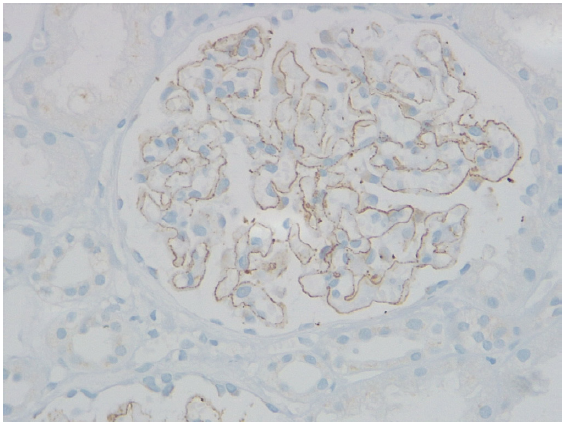
- Neural Epidermal Growth Factor like 1 (NELL1) positivity on immunohistochemistry (IHC).
Three cases were managed with conservative management, and all achieved complete remission (CR). Seven patients required immunosuppression with calcineurin inhibitor (CNI) and steroids for 6–12 months. Of these, two achieved CR, four achieved partial remission (PR), and one was CN-resistant, requiring cyclical cyclophosphamide-corticosteroid. There were no deaths in our study, and treatment-related side-effects included diabetes in one patient and hypertension in another.
Discussion
Our case series adds to the growing literature on NELL1-MN, a relatively new entity and have been linked to cancer, drugs, infections, autoimmune diseases, stem cell transplantation, and use of mercury containing CAM, and skin creams.4-6 In this case series, none of our patients had any signs of malignancy which was similar to a series by Wang et al.7 three (30%) had a history of consuming CAM, although exact nature of medicines could not be delineated. Kurien et al.4 reported that approximately 35% of MN cases in India were associated with traditional indigenous medicine use, of which 88% were NELL1-positive. Another three (30%) had history of prolonged NSAID intake for migraine or as an over-the-counter pain killer. A possible correlation between heavy metals in CAM or skin fairness creams and incidence of NELL1-MN has been reported in various series recently.6,8,9 There was no history of skin fairness cream use in our patients.
In the current study, IgG1 and IgG4 staining was observed in 100% cases, with IgG1 predominance in 20% and IgG1–IgG4 co-dominance in 80%, and segmental IgG staining was observed in 30% and C3 staining in 70% [Figure 2]. Sethi et al.2 reported that partial or segmental IgG1 staining was predominant in NELL1 MN. The results are also similar to Caza et al.,5 who found pure segmental staining (IgG) in 45% of the cases and C3 in three-fourths of cases.
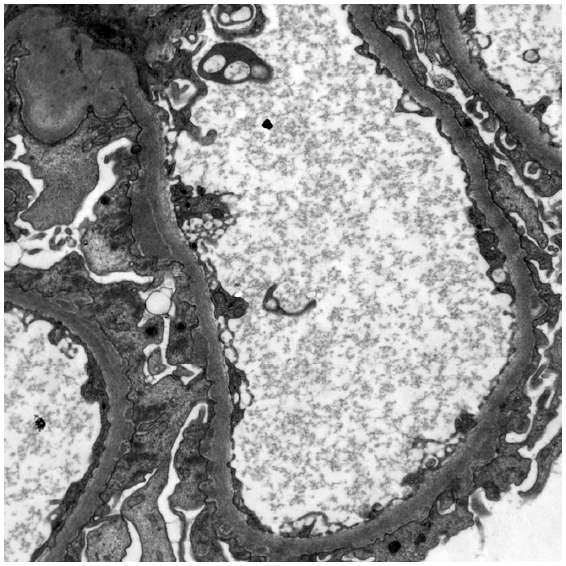
- Electron microscopy showing subepithelial deposits.
Little information is available regarding the optimal treatment and clinical outcomes in patients with non–malignancy-related NELL1-MN. In our study, all but one patient experienced clinical remission with CR in 5 (50%) and PR in 4 (40%). Out of these, three were managed with supportive RAAS inhibition alone and all achieved CR. Those requiring immunosuppression had a mean treatment duration with CNI of 9.3±2.5 months. This was comparable to results of Wang et al.,7 who reported follow-up data for 12 patients, of whom 10 received immunosuppressive therapy, and 11 achieved clinical remission (CR: 5 and PR: 6). In the study by Caza et al.,5 only 15 patients (25.42%) received immunosuppressive therapy, with eight receiving CNI, three cyclophosphamide and mycophenolate mofetil combined with steroids each, and one with rituximab therapy. Sultan et al.8 reported a successful response to cyclical cyclophosphamide-corticosteroids in two patients with skin-whitening cream-associated NELL1-MN. A recent study by Narayanasami et al.10 examined the clinical characteristics and remission rates of NELL1-MN and compared them with unidentified antigen-associated MNs. Immunosuppression was less frequently required in NELL1 group and, there was no difference in remission rates between cyclical cyclophosphamide-corticosteroid and rituximab-treated patients. In present study, one patient (10%) was CNI-resistant, requiring cyclical cyclophosphamide-corticosteroid. This patient did not respond to immunosuppression and had progressive eGFR decline.
Although this study delineates the etiological and longitudinal outcomes of NELL1-MN, it has certain limitations. These encompass a retrospective nature, small sample size, brief follow-up duration, a dearth of information regarding the nature of CAM, non-usage of rituximab, and absence of antibody monitoring.
Nonetheless, the present study suggests that oral CNI with steroids could prove efficacious in managing patients without spontaneous remission or non-responsive to RAAS inhibition, and if ineffective, modified Ponticelli regimen could serve as an effective alternative.
NELL1-associated MN demonstrates an association with CAM and NSAID intake, with possible role of heavy metals, and not with malignancy, as previously thought. Some patients may undergo spontaneous or CR with elimination of inciting factor or effective RAAS inhibition. We document good response to CNI therapy, thus, pointing to avoidance of cytotoxic agents and biologics as first line, to prevent unnecessary side effects. Long-term response, remission, and relapse rates need further studies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Immunological pathogenesis of membranous nephropathy: Focus on PLA2R1 and its role. Front Immunol. 2019;10:1809.
- [CrossRef] [PubMed] [Google Scholar]
- New ‘antigens’ in membranous nephropathy. J Am Soc Nephrol. 2021;32:268-78.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Membranous nephropathy. Nat Rev Dis Primers. 2021;7:69.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Traditional indigenous medicines are an etiologic consideration for NELL1-positive membranous nephropathy. Kidney Int. 2022;102:1424-6.
- [CrossRef] [PubMed] [Google Scholar]
- NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021;99:967-76.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Lipoic acid supplementation associated with neural epidermal growth factor-like 1 (NELL1)-associated membranous nephropathy. Kidney Int. 2021;100:1208-13.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Neural epidermal growth factor-like 1 protein-positive membranous nephropathy in chinese patients. Clin J Am Soc Nephrol. 2021;16:727-35.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mercury-associated neural epidermal growth factor-like 1 protein (NELL-1) positive membranous nephropathy after use of skin lightening creams. Clin Toxicol (Phila). 2023;61:387-91.
- [CrossRef] [PubMed] [Google Scholar]
- Nerve epidermal growth factor-Like 1 protein (NELL-1) expression in mercury-related membranous nephropathy: Is it a true association or a chance occurrence? Indian J Nephrol. 2024;34:482-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical characteristics and outcomes of neural epidermal growth factor-like 1 protein-associated membranous nephropathy. Kidney Int Rep. 2024;9:1513-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







