Translate this page into:
Intraperitoneally Administered Vancomycin Results in Suboptimal Serum and Peritoneal Effluent Drug Levels in Patients with PD-related Peritonitis
Corresponding author: Sreejith Parameswaran, Department of Nephrology, Jawaharlal Institute of Post Graduate Medical Education and Research (JIPMER), JIPMER Campus, Puducherry, India. E-mail: sparameswaran@outlook.com
-
Received: ,
Accepted: ,
How to cite this article: Sampath E, Sejpal KN, Sastry AS, Selvarajan S, Priyamvada PS, Parameswaran S. Intraperitoneally Administered Vancomycin Results in Suboptimal Serum and Peritoneal Effluent Drug Levels in Patients with PD-related Peritonitis. Indian J Nephrol. doi: 10.25259/IJN_59_2024
Abstract
Background
Intraperitoneal vancomycin is commonly used to treat peritoneal dialysis (PD)-related peritonitis. Therapeutic drug level monitoring helps optimize the use of vancomycin in CKD patients. We studied whether sufficient serum levels were achieved in patients with PD-related peritonitis treated with the commonly used dose of vancomycin in patients with end stage renal disease.
Materials and Methods
All consecutive patients with PD-related peritonitis during 19 months were studied. Patients received IP Vancomycin 15 mg/kg Q96H and amikacin 2 mg/kg while awaiting culture reports. Vancomycin concentration in serum and dialysate was determined by a validated liquid chromatography-tandem mass spectrometry assay at 1, 12, 24, and 96 h. The primary outcome was the drug levels in serum and peritoneal fluid, and the secondary outcome was peritonitis treatment failure.
Results
A total of 45 episodes of PD-related peritonitis were treated, of which 41 fulfilled the inclusion criteria and were included in the PK analysis. Recommended serum vancomycin trough level of >15 mg/L was achieved in only two cases. Twenty-one episodes required catheter removal.
Conclusion
In patients with PD-related peritonitis, intermittent intraperitoneal administration of 15 mg/kg of vancomycin every 96 h does not achieve the recommended serum and dialysate levels of vancomycin. There is an urgent need for pharmacokinetic studies on commonly used IP antibiotics in the PD population to facilitate correct dose recommendations.
Keywords
Peritoneal dialysis
Peritonitis
Pharmacokinetics
Vancomycin
Liquid chromatography – Mass spectroscopy
Residual renal function
Minimum inhibitory concentration
Introduction
Peritonitis is a critical complication of peritoneal dialysis (PD) and is associated with significant morbidity and mortality.1,2 Vancomycin is commonly employed in the treatment of PD-related peritonitis. The International Society of Peritoneal Dialysis (ISPD) recommends a dose of 15–30 mg/kg every 5–7 days administered intraperitoneally (IP) Peritoneal membrane characteristics, dwell time, and peritoneal inflammation due to peritonitis affect the bioavailability and equilibration half-life of IP vancomycin.3 Serum vancomycin levels of 15 mg/L or AUC/MIC ratio of 400 is recommended for efficacy and to prevent the development of resistance.4 Therapeutic drug level monitoring (TDM) is not routinely available, and the turnaround time does not permit real-time decision-making. Recommended IP doses of most antibiotics are based on published clinical experience and not pharmacokinetic studies. These recommendations do not consider residual renal function. The importance of residual renal function in antibiotic dosing was highlighted in a prospective study of 339 episodes of peritonitis. Patients with a urinary creatinine clearance of more than 5 mL/min had higher rates of treatment failure.5 We studied whether sufficient serum levels were achieved in patients with PD-related peritonitis treated with the recommended dose of vancomycin.
Materials and Methods
Consecutive patients presenting to the Department of Nephrology during 19 months with PD-related peritonitis were included. Those with malignancy or those who had received antibiotics within 48 h before presentation and aged less than 18 years were excluded. Ethical approval and patient consent was obtained.
Once a diagnosis of peritonitis was made, patients were asked to perform PD exchange and the drain bag was disconnected. Under sterile precautions, three PD effluent samples of 50 mL each were collected. Two of these samples were centrifuged at 3000 rpm for 15 min, one was sent for Gram stain and inoculated in conventional aerobic solid culture media. Sensitivity was analyzed using the Kirby-Bauer disc diffusion method. The second sample was reconstituted, suspended in 10 mL sterile saline, and inoculated in BACTEC BD aerobic blood culture media. The third sample was stored for further microbiologic studies.
Patients were started on an intermittent regimen of empirical antibiotics administered IP. Vancomycin 15 mg/kg Q96H (Vancocin Chandra Bhagat Pharma Pvt. Ltd.) and amikacin 2 mg/kg q24h with a dwell time of 8 h were used. Patients with urine volume >100 mL/day received a 25% higher initial dose of antibiotics.6 Five milliliters of blood samples were collected at 1, 12, 24, and 96 h after the initial administration of antibiotics. Five milliliters of PD effluent fluid was collected 24 and 96 h after administration of vancomycin. Blood samples were settled to clot, centrifuged at 3000 rpm for 10 min, and the serum was separated and stored at −80°C without delay.
Drug estimation
Vancomycin concentration in serum and peritoneal dialysate was determined by a validated liquid chromatography-tandem mass spectrometry assay method using electron spray ionization interface in a positive mode on an Acquity TQ Detector with Alliance e2695 LCMS (Waters). The stationary phase was the XTerra MS C18 column (5 µm, 3.9 × 150 mm), and the mobile phase was methanol and 0.1% formic acid in water in a ratio of 40:60%, respectively, at a flow rate of 0.6 mL/minute. The column temperature was maintained at 30°C, and total run time was 3 min. Neomycin was used as an internal control standard, and the standards for vancomycin and neomycin used were from Sigma-Aldrich laboratories, USA. The primary stock concentration of vancomycin and neomycin (IS) was prepared in methanol at 1 mg/mL. Working stock solution of vancomycin standards was prepared at 100 µg/mL and 10 µg/mL in 50% methanol. Standards of vancomycin were prepared at a concentration of 0.1, 0.2, 0.5, 1, 2, 5, and 8 µg/mL and two quality controls (QCs) at 0.6 and 2.5 µg/mL in matrices of drug-free serum. A simple protein precipitation technique was followed for extraction. Serum (200 µl) was deproteinated with 10% trichloroacetic acid and extracted with 600 µl methanol containing an internal standard (10 µg/mL). Samples were centrifuged at a high speed of 13,500 rpm for 5 minutes, and 20 µl supernatant was injected. The samples were run using Mass Lynx V4.1 software and analyzed using Quanlynx software in LCMS. Linearity was validated from 0.1 to 8 µg/mL. Accuracy and precision were determined from six replicates of QCs at high and low concentrations and were within limits of 10%. For both plasma and peritoneal dialysate, intra-day and inter-day variations were within 15% for both QC levels.
To ensure quality, vancomycin from various manufacturers was purchased from the open market, analyzed for drug concentration, and compared with the brand supplied by our hospital pharmacy for the study.
The primary study outcome was the drug levels in serum and peritoneal fluid. The secondary outcome was treatment failure, defined as catheter removal from refractory peritonitis of recurrent, relapsing, or repeat peritonitis, as defined by the ISPD guidelines.6 Cure was defined as the disappearance of signs of peritonitis with no relapse by day 28 after completion of antibiotics. A complicated episode was defined as the removal of the PD catheter for the resolution of peritonitis. Peritonitis-related death was defined as death either directly or indirectly as a result of peritonitis within 28 days of the onset of illness.
Statistical analysis
Frequencies and percentages were used for categorical variables. Mean ± standard deviation was used for continuous, normally distributed variables, and median (interquartile range; IQR) was used for nonnormally distributed continuous variables. Dichotomous and categorical data were compared using chi-square tests. To compare continuous normally distributed data, the two-tailed unpaired t-test was used, and to compare the continuous nonnormally distributed data, the Mann–Whitney test was used. Univariate logistic regression analysis was used to assess the factors influencing the outcome of peritonitis—cured vs complicated. The impact of serum and peritoneal dialysate vancomycin concentration on the outcomes was compared between the cured and complicated episodes. Serum and PD effluent antibiotic levels were also compared between anuric and nonanuric patients. Two-tailed P values < 0.05 were considered statistically significant. Data were analyzed using the software Statistical Package for Social Sciences version 19.0 (SPSS).
Results
During the study period, there were 45 episodes of PD-related peritonitis, and 41 episodes in 27 patients treated with IP antibiotics were included in the analysis. Figure 1 depicts patient screening, enrollment, and outcomes. The baseline demographic data, etiology of kidney disease, comorbidities, and CAPD treatment details are summarized in Table 1.
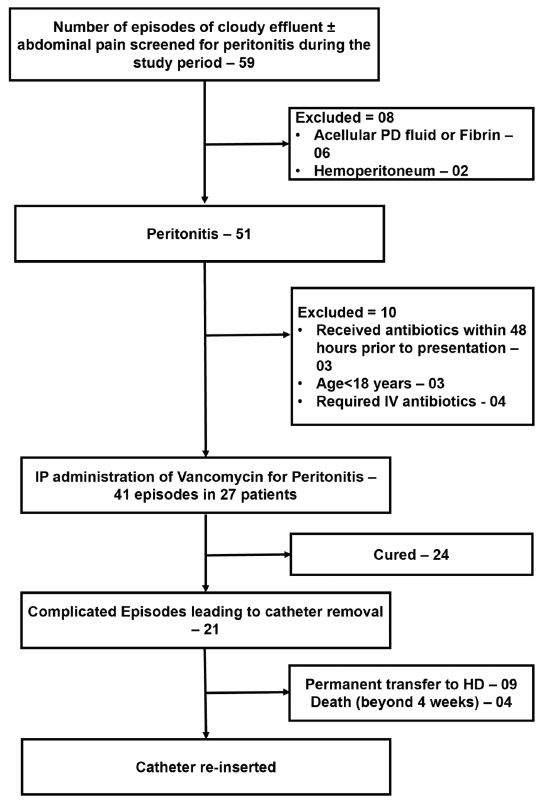
- Patient screening, enrolment and outcomes. PD: peritoneal dialysis, HD: Hemodialysis, IP: Intraperitoneal
| Total number of episodes | 45 |
| Total number of patients | 31 |
| Total number of episodes requiring IP antibiotics | 41 |
| Total number of patients requiring IP antibiotics | 27 |
| Characteristics | n = 31 |
| Age (y) | 39.2 ± 14.8 |
| Male (%) | 16 (51.6) |
| Weight (kg) | 53.9 ± 11.6 |
| Body mass index (kg/m2) | 20.8 ± 2.8 |
| Basic disease (%) | n = 31 |
| Undetermined | 10 (32.2) |
| Chronic glomerulonephritis | 6 (19.3) |
| Focal segmental glomerulosclerosis | 2 (6.5) |
| Diabetic nephropathy | 4 (12.9) |
| HIV-associated nephropathy (HIVAN) | 1 (3.2) |
| Comorbidities | |
| Diabetes mellitus | 6 (19.3) |
| Hypertension | 6 (19.3) |
| Multiple access failures | 2 (6.5) |
| Hepatitis C infection | 2 (6.5) |
| Hemodialysis-associated ascites | 2 (6.5) |
| Post-renal transplant graft loss | 2 (6.5) |
| Coronary artery disease | 1 (3.3) |
| Bronchiectasis | 1 (3.3) |
| PLHA | 1 (3.3) |
| CAPD details | n = 31 |
| Number of exchanges per day | |
| Three | 28 (90.4) |
| Four | 3 (9.6) |
| CAPD duration (median, IQR) months | 15 (7, 28) |
| Nasal staph aureus carrier status (%) | 7 (22.6) |
| Residual renal function (>100 mL) (%) | 17 (55) |
| Caregiver does exchanges | 5 (16.1) |
| Investigations | n = 45 |
| Hemoglobin (g/dL) | 8.6 ± 1.9 |
| Total leukocyte count (×109 cells/L) | 8.23 (5.9, 12.2) |
| Serum albumin (g/dL) | 2.5 ± 0.6 |
| Serum alkaline phosphatase (IU/L) | 257 (191, 377) |
| Random blood sugar (mg/dL) | 97 (85, 119) |
| Blood urea (mg/dL) | 81 ± 34 |
| Serum creatinine (mg/dL) | 8.5 (6.5, 11.1) |
| Serum sodium (mEq/L) | 132.4 ± 5 |
| Serum potassium (mEq/L) | 4 ± 0.66 |
| Serum-corrected calcium (mg/dL) | 8.6 ± 0.9 |
| Serum inorganic phosphorus (mg/dL) | 3.5 ± 1.1 |
| Serum intact PTH (pg/mL) | 226.1 (137.4, 473.4) |
| Serum vit D level (ng/mL) | 17.7 (14.3, 19.9) |
| Serum ferittin (ng/mL) | 683 (457.6, 1241.9) |
| CAPD fluid cell count (First day) (cells/cmm) | 1000 (410, 1700) |
| Hypokalemia was present in 7 (15.6%) of the episodes | |
IP: Intraperitoneal, PLHA: People Living With HIV-AIDS, CAPD: continuous ambulatory peritoneal dialysis, IQR: Inter-quartile range, PTH: Parathyroid hormone
Culture yield and antibiotic sensitivity
The BACTEC system yielded growth (culture positive) in 64.5% of instances of PD peritonitis; 24.4% were gram-positive bacteria, and 34.2% were gram-negative bacteria. The microbiological spectrum is provided in Table 2.
| Total number of episodes | n = 45 |
| Culture negative | 16 (35.5%) |
| Culture positive | 29 (64.5%) |
| Gram-positive organisms | 11 (24.4%) |
| Coagulase negative Staphylococci | 10 (22.2%) |
| Methicillin-resistant Staphylococcus aureus | 1 (2.2%) |
| Gram-negative organisms | 12 (26.6%) |
| Klebsiella pneumonia | 5 (11.1%) |
| Escherichia coli | 3 (6.7%) |
| Pseudomonas sp | 3 (6.7%) |
| Enterobacter sp | 1 (2.2%) |
| Polymicrobial | 3 (6.6%) |
| Acinetobacter lwoffii and Fungal | 1 (2.2%) |
| Klebsiella pneumoniae and Acinetobacter baumanii | 1 (2.2%) |
| Enterococcus fecalis and Pseudomonas stutzeri | 1 (2.2%) |
| (Denovo) Fungal | 3 (6.6%) |
| Candida sp | 2 (3.4%) |
| Penicillium sp | 1 (2.2%) |
Tubercular Peritonitis- TB peritonitis was suspected in four patients who presented with repeated episodes of peritonitis. AFB staining, Lowenstein Jensens medium culture, and TB DNA PCR in the centrifuged specimens were negative for tuberculosis. One patient who presented with recurrent and refractory peritonitis had tuberculous peritonitis, which was diagnosed by omental biopsy during reinsertion of CAPD catheter. PCR: Polymerase Chain Reaction CAPD: Continuous Ambulatory Peritoneal Dialysis
Of the 11 peritonitis episodes from gram-positive bacteria, 10 were coagulase negative staphylococci and 1 was MRSA. All coagulase-negative staphylococci were susceptible to oxacillin except one, which was methicillin-resistant; the MRSA was vancomycin-sensitive. The most common gram-negative bacteria were Klebsiella pneumonia. All gram-negative bacteria showed 100% sensitivity to aminoglycosides (amikacin and gentamicin).
Vancomycin levels in serum and PD effluent
The serum levels of vancomycin were available at 1 h, 12 h, 24 h, and 96 h, and the PD fluid levels were available at 24 h and 96 h, as described in Table 3. The drug levels of vancomycin achieved, and the recommended MIC and trough levels are depicted in Table 4.
| Peritonitis episodes included | n = 41 episodes (27 pts) |
| Age (y) | 39.21 ± 12.75 |
| Male (%) | 12 (44.4%) |
| Weight (kg) | 53.9 ± 11.6 |
| IP vancomycin dose (Mean ± SD) | 953.6 ± 230.3 mg |
| Therapeutic drug levels attained in the serum and PD fluid effluent | |
| Vancomycin serum at 1 h (Median, IQR) | 2.9 (1.5, 4.1) µg/mL |
| Vancomycin serum at 12 h (Median, IQR) | 3.3 (2.5, 4.7) µg/mL |
| Vancomycin serum at 24 h (Median, IQR) | 2.7 (1.7, 4.1) µg/mL |
| Vancomycin serum at 96 h (Median, IQR) | 2.2 (1.3, 2.8) µg/mL |
| Vancomycin PD at 24 h (Median, IQR) | 1.5 (0.9, 2.31) µg/mL |
| Vancomycin PD at 96 h (Median, IQR) | 0.97 (0.75, 1.5) µg/mL |
IP: Intraperitoneal, PD: Peritoneal Dialysis, IQR: Inter-quartile range
| Recommended (for vancomycin) | |
| MIC of vancomycin for MRSA | >4 µg/mL |
| MIC of vancomycin for MRCoNS | 1.5–2 µg/mL |
| Vancomycin therapeutic levels | >15 µg/mL |
| This study | n = 41 |
| Serum peak level >15 µg/mL at 12 h | 2 (19.2 and 47.4) |
| Serum trough level 12–15 µg/mL at 96 h | Nil (one patient–11.25) |
| PD effluent > 4 µg/mL at 24 h | 6 (14%) |
| PD effluent > 4 µg/mL at 96 h | 3 (7%) |
| PD effluent > 2 µg/mL at 24 h | 26 (64%) |
| PD effluent > 2 µg/mL at 96 h | 4 (10%) |
MRSA: Methicillin Resistant Staphylococcus aureus, MRCoNS: Methicillin Resistant Coagulase Negative Staphylococcus, PD: Peritoneal Dialysis, MIC: Minimum Inhibitory Concentration
In 36.5% of the 41 episodes, residual renal function was lost. Though the nonanuric group (26 episodes, 63.5%) received a higher dose of vancomycin [(1048 ± 227.8) vs (790 ± 166); P value:0.0005], the serum and PD effluent levels at 1 h, 12 h, 24 h, and 96 h were comparable to the anuric group in univariate analysis [Supplementary Table 1].
The dose administered and the drug levels in the serum and PD effluent in both the cured and the complicated groups were comparable [Supplementary Table 2]. In univariate analysis, no statistical significance was demonstrated between these two groups. One patient had transient pruritus following the first dose of IP vancomycin, which subsided on its own.
Table 5 summarizes the outcomes of peritonitis. There was no significant association between the clinical outcomes and whether the recommended trough vancomycin levels were attained in serum or peritoneal effluent.
| Outcomes | n = 45 (31 patients) |
| Cured | 24 (53.4%) |
| Complicated (requiring catheter removal) | 21 (46.6%) |
| Refractory peritonitis | 17 (37.7%) |
| Recurrent peritonitis | 1 (2.2%) |
| Repeat peritonitis | 1 (2.2%) |
| Relapsing peritonitis | 2 (4.3%) |
| Permanent transfer to hemodialysis | 10 (22.2% episodes) (32.2% of patients) |
| Death | 0 |
| Reinsertion of PD catheter | 8 episodes (in 7 patients) (38%) |
PD: Peritoneal Dialysis
Discussion
In patients with PD-related peritonitis treated with IP vancomycin dose of 15 mg/kg Q96H, we found that the serum and PD fluid levels at 1, 12, 24, and 96 h were deficient compared to the recommended trough levels.
Vancomycin undergoes negligible metabolism, and its elimination is mainly through glomerular filtration. This leads to a significant reduction in vancomycin clearance in patients with CKD, resulting in an elimination half-life of 7.5 days in contrast to 4–6 h in patients with normal GFR. The amount of vancomycin reaching systemic circulation from the peritoneal cavity relative to intravenous dosing is 50% in normal subjects and 70–90% in patients with peritonitis.7 The serum level of vancomycin during treating an episode of PD peritonitis depends on peritoneal solute transfer rate, PD prescription, RRF, and other patient characteristics such as weight.3
Our patients generally have a low lean body mass (mean body weight was 55 kg), and the standard prescription was three PD exchanges per day. This is different from the practice in high-income countries where most patients perform four PD exchanges daily and have relatively higher body weight compared to our patient population. The dose of vancomycin used in patients with End Stage Renal Disease (ESRD) and either on HD or PD at our center was 15mg/kg Q96H in the absence of access to TDM. We wanted to test whether this dose achieved therapeutic MIC in our patients with PD-related peritonitis.
The therapeutic peak level so far from published literature varies from 15 to 30 µg/mL against a dose of 15–30 mg/kg measured at 4 h, 6 h, 48 h.8,9 In a recent retrospective cohort study from a single center, a loading dose of 2 g of IP vancomycin led to sub-therapeutic drug levels in one-third of the patients. GFR and weight were independently associated with a likelihood of lower therapeutic levels, and the possibility of achieving a therapeutic level was approximately four times higher with an initial dose of 25 mg/kg compared to <25 mg/kg of dosing. The trough levels in PD fluid range from >2 µg/mL after the first exchanges to 7.7–8.7 µg/mL on the fifth day or seventh day with similar dosing regimens.9,10
In a study by Mulhern et al., a trough level of >12 µg/mL at the end of the first week predicted less number of relapses after PD-related peritonitis (0/13 episodes) compared to levels <12 µg/mL (9/13 episodes) among 31 peritonitis patients on IV vancomycin 15 mg/kg every seventh day.11
It is a matter of concern that most patients did not attain the MIC of >15 mcg/mL in our study. This was very unlikely due to the poor quality of drugs as we tested the study drug from the hospital pharmacy against five other popular brands procured from the open market. We did not analyze transport status in our patients because the transport parameters during an episode of peritonitis are expected to be different from when there was no active peritoneal infection. Our patients had a mean body weight of 55 kg, we accounted for RRF in determining the drug dose, and our patients were performing only three PD exchanges per day, potentially reducing peritoneal drug clearance. The blood and peritoneal fluid samples collected were stored at −80°C, and analysis was performed every month. Vancomycin is known to remain stable without degradation for up to 85 days when stored at minus 85°C.12 Despite all these, the observed serum and peritoneal drug levels were deficient. All these suggest that the dose of vancomycin used was insufficient, and a higher dose or more frequent dosing interval is likely required to achieve serum levels of >15 mcg/mL in patients with PD-related peritonitis.
Despite the deficient serum and peritoneal effluent levels of vancomycin, we did not find any correlation between the drug levels and clinical outcomes and the incidence of complications. This is likely because gram-positive organisms accounted for only 11 episodes (24.4%) of peritonitis, all of which, except for one, were from MSSA. The only episode of MRSA peritonitis was refractory and resulted in catheter removal. The MIC for MSSA is less than 15 mcg/mL. The rest of the infections were from gram-negative organisms, fungi, or from mycobacteria. In this regard, it is emphasized that the study was designed primarily to assess the drug levels from the commonly used dose of vancomycin and not to analyze the association of drug levels with clinical outcomes.
Our study had some limitations. Serum samples were collected only at 1, 12, 24, and 96 h, precluding the calculation of AUC for vancomycin. The reason why vancomycin peak level monitoring was set at 1 h and 12 h, respectively, after IP administration, was based on earlier pharmacokinetics studies to document the peak levels. Samples were collected at 24 and 96 h to assess the trough levels. More frequent samples would have required hospitalization of the patients which was logistically challenging. A limited sampling strategy was designed to allow studying the peak and trough levels and also to avoid multiple venipunctures and inconvenience to the patient. The first sample was collected at 1 h after IP administration of vancomycin in our study. This might have been too early to study the peak level. When administered IP, the equilibration half-life of vancomycin in PD patients with peritonitis was 1.6 to 2.9 h, and it was 2.9 h in those without peritonitis.3 We used a vancomycin dose of 15 mg/kg (instead of a higher dose); however, this was in line with the standard practice at our center and most other centers in our region where TDM is not freely available or accessible. Our primary intention was to check whether the commonly employed dose at our center was appropriate. Characteristics and PD prescriptions in our patients were different from those in most high-income countries, and findings may not be generalizable to all PD patients.
Based on our observations, it may be safe to conclude that in the absence of TDM, using a fixed dose of IP vancomycin 15 mg/kg Q96H is likely to be ineffective in treating MRSA. More studies on AUC of vancomcyin with various doses need to be undertaken to improve the vancomycin dose recommendation in treating PD-related peritonitis. TDM should be more widely used in monitoring vancomycin treatment in this situation until more data are available on the dosage of vancomycin required to attain an AUC of 400–600 mg·h/L without TDM.
Conclusion
In patients with PD-related peritonitis, intermittent intraperitoneal administration of 15 mg/kg of vancomycin every 96 h does not attain the recommended serum and peritoneal effluent levels of vancomycin in the treatment of MRSA. Therapeutic drug monitoring may be necessary to ensure sufficient serum levels of the drug. There is an urgent need for pharmacokinetic studies on commonly used IP antibiotics in the PD population to facilitate correct dose recommendations based on scientific information.
Acknowledgment
JIPMER provided the intramural funds to undertake this research project. We are grateful to Mr. Rajan Sundaram, technical assistant, Department of Pharmacology, for his excellent work and support on drug level estimation.
Conflicts of interest
There are no conflicts of interest.
References
- Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit Dial Int. 2011;31:651-62.
- [CrossRef] [PubMed] [Google Scholar]
- Recent peritonitis associates with mortality among patients treated with peritoneal dialysis. J Am Soc Nephrol JASN. 2012;23:1398-405.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Vancomycin in peritoneal dialysis: Clinical pharmacology considerations in therapy. Perit Dial Int J Int Soc Perit Dial. 2020;40:384-93.
- [CrossRef] [Google Scholar]
- The role of monitoring vancomycin levels in patients with peritoneal dialysis-associated peritonitis. Perit Dial Int J Int Soc Perit Dial. 2015;35:222-8.
- [CrossRef] [Google Scholar]
- Residual kidney function and peritoneal dialysis–associated peritonitis treatment outcomes. Clin J Am Soc Nephrol CJASN. 2017;12:2016-22.
- [Google Scholar]
- Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30:393-423.
- [CrossRef] [PubMed] [Google Scholar]
- Absorption of intraperitoneal antibiotics. Drug Intell Clin Pharm. 1988;22:58-61.
- [CrossRef] [Google Scholar]
- Clearance from dialysate and equilibration of intraperitoneal vancomycin in continuous ambulatory peritoneal dialysis. Clin Pharmacokinet. 1990;18:485-90.
- [CrossRef] [Google Scholar]
- Comparative study of intraperitoneal and intravenous vancomycin pharmacokinetics during continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1987;31:173-7.
- [CrossRef] [Google Scholar]
- Intraperitoneal vancomycin concentrations during peritoneal dialysis–associated peritonitis: Correlation with serum levels. Perit Dial Int J Int Soc Perit Dial. 2012;32:332-8.
- [Google Scholar]
- Trough serum vancomycin levels predict the relapse of gram-positive peritonitis in peritoneal dialysis patients. Am J Kidney Dis Off J Natl Kidney Found. 1995;25:611-5.
- [Google Scholar]
- Stability of vancomycin 25 mg/mL in ora-sweet and water in unit-dose cups and plastic bottles at 4°C and 25°C. Can J Hosp Pharm. 2010;63:366-72.
- [PubMed] [Google Scholar]








