Translate this page into:
Is Ambulatory Blood Pressure Monitoring Required for Elderly Hemodialysis Patients during the Interdialytic Period? - Experience of a Tertiary Care Center in South India
Address for correspondence: Dr. E. Indhumathi, Associate Professor, Department of Nephrology, Sri Ramachandra Institute of Higher Education and Research (Deemed to be University), No 1, Ramachandra Nagar, Porur, Chennai - 600 116, Tamil Nadu, India. E-mail: drindhusrmc@yahoo.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Hypertension (HT) is a common and challenging problem in patients on dialysis. Routine peri-dialytic blood pressure (BP) recordings are unable to diagnose HT accurately and stratify cardiovascular risk. We report here an analysis of 2 years, single-center experience on 24-hour ambulatory blood pressure monitoring (ABPM) in elderly hemodialysis patients in the interdialytic period.
Materials and Methods:
Data of all the patients above 65 years of age undergoing hemodialysis between November 2017 and December 2019 in our hemodialysis unit and for whom 24-hour ABPM was done were collected. Demographics, clinical profile, pre- and post-dialysis BP recordings, 24-hour ABPM characteristics, and the outcome status were analyzed.
Results:
Of the 37 patients, 28 (75.7%) were males with a mean age of 67.73 years; 67.6% were diabetic. HT was found in all patients (100%), and uncontrolled HT was noted in 30 (81%) patients by ABPM. Patients with uncontrolled HT were also nondippers of BP (100%). A significant association was observed between nondippers and coronary artery disease (n = 27, 90%, P = 0.004). Masked HT was found in 9 (24.3%) patients with normal peridialytic BP (n = 9, 24.3%, P = 0.000). No significant difference was noted between diabetic and nondiabetic patients regarding dipping status or mortality. Among 37 patients, 9 (24.3%) died during follow-up with uncontrolled HT as a significant risk factor (P = 0.05).
Conclusion:
The prevalence of uncontrolled HT with blunted circadian rhythm was high as detected by ABPM in the interdialytic period among elderly hemodialysis patients and had a significant impact on mortality. Masked uncontrolled HT as measured by ABPM was not uncommon in patients with normal peridialytic BP.
Keywords
ABPM
elderly
hemodialysis
hypertension
Introduction
Hypertension (HT) is a common and challenging problem in patients on chronic hemodialysis (HD). HT in dialysis has several unique features. First, the prevalence is very high with 75% to 90% of patients on HD being hypertensive.[1] Second, young HD patients have higher average systolic blood pressure (SBP) than the elderly, perhaps because arterial stiffening occurs at a younger age, and the elderly HD patient has an increased risk for cardiomyopathy, unlike the general population in which SBP increases with age.[2]
In the general population, a linear relationship exists between blood pressure (BP) and mortality.[34] In contrast, a U-shaped curve or a reverse J-shaped curve of association of BP occurs with the survival of patients on dialysis. Patients with low SBPs (<110 mmHg) have higher mortality and no deleterious effects observed until SBP reached >180 mmHg. Thus, high BP in chronic dialysis is associated with adverse outcomes, low BP has an even stronger association with mortality.[5] The National Kidney Foundation's Kidney Disease Outcomes and Quality Initiative (KDOQI) 2005 guidelines recommended aiming for a predialysis BP of 140/90 mmHg and a postdialysis BP of 130/80 mmHg.[6] Blood pressure variability occurs over the dialysis session because of volume shift, and hence peridialytic BP recordings are inaccurate compared with interdialytic BP recordings.[78]
In a multicenter study using peridialytic BP recording, among 89% of patients on antihypertensive, only 30% had control of HT. Therefore, in the absence of accurate measurement of BP in the interdialytic period, a large number of patients will be falsely diagnosed with controlled HT.[9]
The relationship between elevated BP recorded in the interdialytic period and mortality is direct and linear as in the general population.[10] Compared with peridialytic and home BP recordings, 24-hour ambulatory BP displays closer associations with indices of target organ damage and is a stronger predictor of mortality.[811] Interdialytic BP measurement is superior for diagnosing masked HT[12] and nocturnal HT, which is associated with target organ damage and increased cardiovascular (CV) events.[813] Therefore, ambulatory blood pressure monitoring (ABPM) is considered the “gold standard” approach for the management of HT among patients on dialysis.[714]
Here, we analyzed the interdialytic BP pattern, nocturnal BP dipping status, masked uncontrolled hypertension (MUCH), and factors associated with all-cause mortality of elderly HD patients for whom 24-hour ABPM was done.
Materials and Methods
Data of elderly patients undergoing HD for whom 24-hour ABPM was done between November 2017 and December 2019 in a tertiary care hospital dialysis center were collected. Among 41 patients, only 37 patients were included for analysis because BP recordings were inadequate in two patients and two had withdrawn from the dialysis. Patients undergoing dialysis for more than 3 months and 8 to 12 hours/week were included for evaluation. Routine laboratory investigations taken before dialysis within 4 weeks of ABPM measurements and echocardiography done postdialysis were taken for analysis. Adequacy of dialysis was assessed by online Kt/V done during the week of ABPM measurement.
Blood pressure
Routinely predialysis BP was measured manually using an aneroid sphygmomanometer just before the initiation of dialysis in the dialysis unit by dialysis staff members, every half an hour during dialysis, and at the end of the dialysis. The average BP over 2 weeks was taken as predialysis and postdialysis BP for the evaluation. As per the KDOQI guidelines, a predialysis BP of >140/90 mmHg and a postdialysis BP of >130/80 mmHg are considered uncontrolled BP.
ABPM was done for all patients using Meditech ABPM-05 device for 24 hours on nondialysis days. Although the recording of ABPM in the interdialytic period for 44 hours is better, for the convenience of the patient and better compliance, 24-hour monitoring was done in our dialysis unit. The monitor was placed on the nonfistula arm. Patients were asked to follow their routine activities and to take regular antihypertensive drugs. BP was measured automatically every 15 minutes in the active period (6 a.m. to 10 p.m.) and every 30 minutes during the passive period (10 p.m. to 6 a.m.). All patients had a minimum of 85% of the total readings. The recordings were downloaded using the manufacturer's software, and the data were analyzed further.
Daytime or active-period SBP ≥135 mmHg was considered as uncontrolled BP and nighttime or passive-period BP ≥120 mmHg was considered as uncontrolled BP. Nocturnal dipping status of BP was defined when the fall in average nighttime SBP was more than 10% of average daytime BP. It was calculated as (average nighttime SBP/average daytime SBP) × 100 and expressed as a percentage. Patients with less than 90% are dippers, more than 90% but less than 100% are defined as nondippers, and more than 100% as reverse dippers.
Statistical analysis
Data analysis was done using Statistical Package for the Social Sciences (SPSS) Version 17. All categorical variables were expressed as numbers and percentages, continuous variables as mean ± standard deviation (SD). Values between the two groups were compared by Student's t test or by the Mann–Whitney U test, as appropriate. A Chi-square test was used for comparison between categorical variables. Univariate Cox regression analyses were performed with mortality as the outcome variable. All statistical tests performed were two tailed, and a significance level of P < 0.05 was considered as statistically significant.
Results
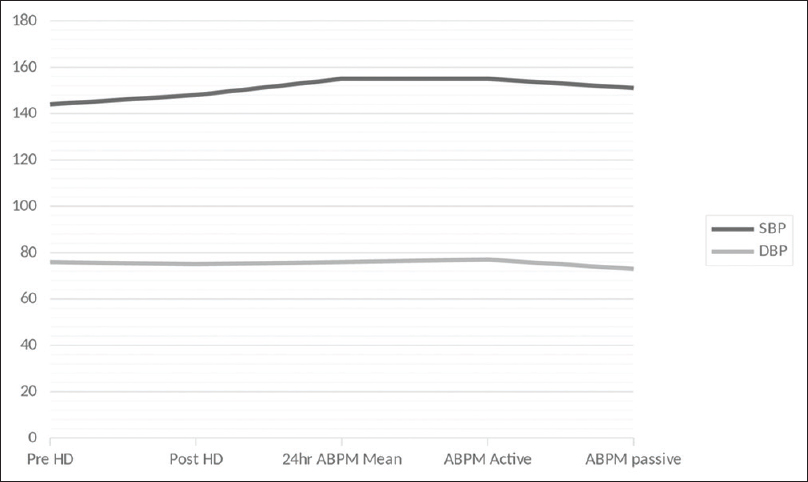
Data of a total of 37 elderly dialysis patients in whom 24-hour ABPM was done were analyzed. Clinical, demographic, and lab investigations are given in Table 1. The mean age was 67.73 ± 3.36, and the majority of them were (75.7%) males. Diabetes was present in 67.6%, HT in 75.7%, and coronary artery disease (CAD), a common comorbid condition in our population, in 81.1%. The average duration of dialysis was 26.64 months, and most of them were adequately dialyzed (Kt/V = 1.27). Mean predialysis systolic and diastolic BP were 144.05 ± 10.91 mmHg and 76.27 ± 8.53 mmHg, respectively. The mean postdialysis SBP was 148.38 ± 13.02 and diastolic BP was 74.76 ± 7.38 mmHg as shown in Figure 1.
| Variables | Mean/percentage |
|---|---|
| Mean age (years) | 67.73±3.36 |
| Malea | 28 (75.7%) |
| Diabetes mellitusa | 25 (67.6%) |
| Hypertensiona | 28 (75.7%) |
| Coronary artery diseasea | 30 (81.1%) |
| Dialysis Vintage (months)b | 24.64 |
| Kt/Vb | 1.27±0.027 |
| Hb (g/dL)b | 9.07±1.75 |
| Albumin (g/dL)b | 3.60±0.50 |
| Calcium (mg/dL)b | 8.44±1.22 |
| Phosphorus (mg/dL)b | 4.06±2.04 |
| Uric acid (mg/dL)b | 5.46±1.55 |
| iPTH (pg/mL)b | 143.05±52.95 |
| Pre-dialysis Systolic BP (mmHg)b | 144.05±10.92 |
| Pre-dialysis Diastolic BP (mmHg)b | 76.27±8.52 |
| Post-dialysis Systolic BP (mmHg)b | 148.38±13.02 |
| Post-dialysis Diastolic BP (mmHg)b | 74.76±7.38 |
| ABPM Systolic BP (mmHg)b | 155.02±20.96 |
| ABPM Diastolic BP (mmHg)b | 75.94±11.63 |
| ABPM MAP (mmHg)b | 102.94±13.69 |
| ABPM Active Systolic BP (mmHg) | 154.99±20.23 |
| ABPM Active Diastolic BP (mmHg) | 76.55±10.788 |
| ABPM Active MAP | 103.36±12.79 |
| ABPM passive Systolic BP (mmHg) | 151.47±20.75 |
| ABPM passive Diastolic BP (mmHg) | 73.35±9.974 |
| ABPM passive MAP | 95.79±14.3995 |
| Dippersa | 7 (18.9%) |
| Non-Dippersa | 30 (81.1%) |
| Mortalitya | 9 (24.3%) |
a Number and percentage, b mean and standard deviation. ABPM: Ambulatory blood pressure monitoring, BP: Blood pressure, iPTH: Intact parathyroid hormone, MAP: Mean arterial pressure
- Mean blood pressure characteristics of peridialytic and interdialytic ABPM (ambulatory blood pressure monitoring)
ABPM mean SBP was 155.02 ± 20.96 mmHg and diastolic BP was 75.94 ± 11.63 mmHg with a mean arterial pressure (MAP) of 102.94 ± 13.69 mmHg. A total of 28 patients (75.7%) were known hypertensive taking antihypertensive drugs. Using ABPM, all 37 patients (100%) were found to have HT, thus unmasking HT in nine (24.3%). There is a high prevalence of uncontrolled HT in our cohort (n = 30, 81%). All the patients with uncontrolled ABPM SBP had a nondipping pattern of BP. Thus, 81% of elderly patients in our dialysis unit were nondippers. Nine patients with normal peridialytic BP were found to have uncontrolled HT by ABPM as well as nondippers of nocturnal BP (P = 0.000). Demographic, clinical, and laboratory factors influencing the nocturnal dipping status of BP and the various ABPM characteristics are given in Table 2. Age, gender, dialysis vintage, diabetic status, and adequacy of dialysis were not correlated with nocturnal dipping status. CAD was significantly associated with nondipper state (n = 27, 90%, P = 0.004). None of the patients with nondipper status had postdialytic diastolic BP more than 85 mmHg (P = 0.036). In the nondipping group, only 10% had ABPM active period/daytime uncontrolled diastolic BP (P = 0.034). Significant variables associated with the nondipping pattern of BP are shown in Figure 2.
| Variables | Non-Dipper (n=30) | Dipper (n=7) | P |
|---|---|---|---|
| Mean age (years)b | 67.83±3.61 | 67.29±2.13 | 0.70 |
| Malea | 24 (80%) | 4 (57.1%) | 0.20 |
| Diabetes mellitusa | 20 (66.7%) | 5 (71.4%) | 0.80 |
| Hypertension | 23 (76.7%) | 5 (71.4%) | 0.77 |
| Coronary artery diseasea | 27 (90%) | 3 (42.9%) | 0.004 |
| Dialysis vintage (months)b | 24.46 | 26.66 | 0.47 |
| Kt/Vb | 1.25±0.21 | 1.36±0.14 | 0.23 |
| Hb (g/dl)b | 8.89±1.72 | 9.87±1.72 | 0.18 |
| Calcium (mg/dL)b | 8.38±1.328 | 8.68±0.61 | 0.56 |
| Uric acid (mg/dL)b | 5.51±1.62 | 5.21±1.29 | 0.65 |
| iPTH (pg/mL)b | 145.44±55.82 | 134.54±40.57 | 0.63 |
| Pre-dialysis Systolic BP (mmHg)b | 145.33±10.41 | 138.57±12.15 | 0.14 |
| Pre-dialysis Diastolic BP (mmHg)b | 76.40±7.45 | 75.71±12.93 | 0.85 |
| Post-dialysis Systolic BP (mmHg)b | 148±12.14 | 150±17.32 | 0.72 |
| Post-dialysis Diastolic BP (mmHg)b | 74.67±6.31 | 75.14±11.59 | 0.88 |
| Ambulatory BP | |||
| 24-h mean Systolic BP (mmHg)b | 155.32±18.08 | 153.70±32.44 | 0.85 |
| 24-h mean Diastolic BP (mmHg) | 75.12±9.959 | 79.45±17.738 | 0.383 |
| 24-h MAP (mmHg) | 101.72±11.647 | 108.15±20.742 | 0.26 |
| 24-h Systolic BP >135 mmHga | 26 (86.7%) | 4 (57.1%) | 0.05 |
| 24-h Diastolic BP >85 mmHga | 4 (13.3%) | 3 (42.9%) | 0.05 |
| Active period Systolic BP (mmHg)b | 154.4±16.35 | 157.51±33.9 | 0.72 |
| Active period Diastolic BPb | 75.02±8.861 | 83.12±16.019 | 0.073 |
| Active period MAP (mmHg)b | 101.52±10.329 | 111.29±19.38 | 0.068 |
| Active period Systolic BP >135 mmHga | 27 (90%) | 5 (71.4%) | 0.19 |
| Active period Diastolic BP >85 mmHga | 3 (10%) | 3 (42.9%) | 0.03 |
| Passive period Systolic BP (mmHg)b | 155.07±19.54 | 136.03±19.84 | 0.02 |
| Passive period Diastolic BP (mmHg)b | 73.87±9.39 | 71.16±12.79 | 0.52 |
| Passive period MAP (mmHg)b | 97.55±12.72 | 88.214±19.46 | 0.12 |
| Passive period Systolic BP >135 mmHga | 26 (86.7) | 3 (42.9) | 0.01 |
| Passive period Diastolic BP >85 mmHga | 3 (10) | 1 (14.3) | 0.74 |
| Mortalitya | 7 (23.3%) | 2 (28.6%) | 0.77 |
a Number and percentage, b mean and standard deviation. ABPM: Ambulatory blood pressure monitoring, BP: Blood pressure, iPTH: Intact parathyroid hormone, MAP: Mean arterial pressure
- Significant association of variables with nondippers
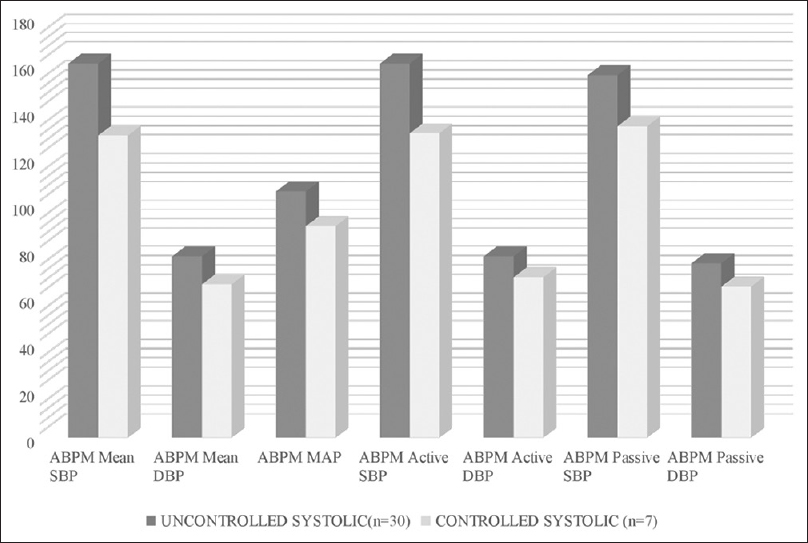
Patients with uncontrolled HT were noted to have significantly elevated ABPM active period: SBP (P = 0.0001), diastolic BP (P = 0.04), and MAP (P = 0.01), as well as passive period SBP (P = 0.011) and diastolic BP (P = 0.01) as shown in Figure 3. CAD was significantly associated with uncontrolled HT (n = 26, 87.3% P = 0.05). A detailed description of the characteristics between uncontrolled and controlled HT is given in Table 3. Of the 37 patients, nine (24.3%) died during the study period. Although patients who all died had uncontrolled HT, none of the patients with controlled HT had any mortality as shown in Table 4. CV events were the common cause of death in four patients (44.4%), sepsis in four (44.4%), and stroke in one (11.1%). No significant difference was noted between the diabetic and nondiabetic groups regarding control of blood pressure, dipping status, and mortality.

- Interdialytic ABPM (ambulatory blood pressure monitoring) systolic blood pressure (BP) – Uncontrolled BP versus controlled BP
| Variables | Uncontrolled SBP (≥135) n=30 | Controlled SBP (<135) n=7 | P |
|---|---|---|---|
| Age in years | 67.87±3.451 | 67.14±3.132 | 0.61 |
| Male | 22 (73.3) | 6 (85.7%) | 0.49 |
| DM | 20 (66.6%) | 5 (71.4%) | 0.42 |
| CAD | 26 (86.7) | 4 (57.1) | 0.05 |
| Pre-dialysis Systolic BP (mmHg) | 145±11.371 | 140±8.165 | 0.28 |
| Pre-dialysis Diastolic BP (mmHg) | 73.2±7.471 | 68±8.246 | 0.09 |
| Pre-dialysis Systolic BP >135 mmHg | 24 (80) | 5 (71.4) | 0.62 |
| Pre-dialysis Diastolic BP >85 mmHg | 2 (6.7) | 0 | 0.48 |
| Post-dialysis Systolic BP (mmHg) | 146.67±13.113 | 139.57±6.901 | 0.08 |
| Post-dialysis Diastolic BP (mmHg) | 68.4±6.179 | 67.71±8.44 | 0.42 |
| Post-dialysis Systolic BP >130 mmHg | 27 (90) | 5 (71.4) | 0.19 |
| Post-dialysis Diastolic BP >80 mmHg | 1 (3.3) | 0 | 0.62 |
| ABPM Systolic BP (mmHg) | 160.97±18.368 | 129.5±8.268 | 0.0001 |
| ABPM Diastolic BP (mmHg) | 78.3±10.948 | 65.86±9.279 | 0.009 |
| ABPM Diastolic BP >85 mmHg | 7 (23.3) | 0 | 0.15 |
| ABPM MAP | 105.75±12.641 | 90.88±11.975 | 0.008 |
| ABPM PP | 82.68±12.789 | 72.35±13.274 | 0.06 |
| ABPM Active Systolic BP (mmHg) | 160.63±17.397 | 130.8±12.292 | 0.0001 |
| ABPM Active Diastolic BP (mmHg) | 78.23±10.338 | 69.39±10.381 | 0.049 |
| ABPM Active Systolic BP >135 mmHg | 30 (100) | 2 (28.6) | 0.0001 |
| ABPM Active Diastolic BP >85 mmHg | 6 (20) | 0 | 0.19 |
| ABPM Active MAP | 105.78±11.932 | 93.01±11.793 | 0.015 |
| ABPM Active PP | 82.93±13.333 | 72.25±14.809 | 0.070 |
| ABPM Passive Systolic BP (mmHg) | 155.55±19.502 | 133.96±17.439 | 0.011 |
| ABPM Passive Diastolic BP (mmHg) | 75.21±8.808 | 65.39±11.43 | 0.017 |
| ABPM Passive Systolic BP >135 mmHg | 27 (90) | 2 (28.6) | 0.0001 |
| ABPM Passive Diastolic BP >85 mmHg | 4 (13.3) | 0 | 0.30 |
| ABPM Passive MAP | 97.817±14.2518 | 87.101±12.3987 | 0.076 |
| ABPM Passive PP | 81.493±13.2862 | 71.853±10.9525 | 0.08 |
| Mortality | 9 (30%) | 0 | 0.09 |
ABPM: Ambulatory blood pressure monitoring, BP: Blood pressure, CAD: Coronary artery disease, MAP: Mean arterial pressure
| Variables | Non survivors (n=9) | Survivors (n=28) | P |
|---|---|---|---|
| Mean age (years)b | 68.67±4.062 | 67.43±3.132 | 0.34 |
| Malea | 8 (88.9%) | 20 (71.4%) | 0.28 |
| Diabetes mellitusa | 7 (77.7%) | 18 (64.3%) | 0.45 |
| Hypertensiona | 7 (77.8%) | 21 (75%) | 0.86 |
| Coronary artery diseasea | 8 (88.9%) | 22 (78.6%) | 0.49 |
| Left ventricle dysfunctiona | 2 (22.2%) | 2 (7.1%) | 0.20 |
| Kt/Vb | 1.2667±0.16560 | 1.2768±0.22 | 0.90 |
| Hb (g/dL)b | 8.856±2.2495 | 9.150±1.59 | 0.66 |
| Albumin (g/dL)b | 3.344±0.4447 | 3.684±0.49 | 0.05 |
| Calcium (mg/dL)b | 8.3±0.6124 | 8.483±1.37 | 0.70 |
| Phosphorus (mg/dL)b | 4.556±3.904 | 3.904±1.99 | 0.41 |
| Uric acid (mg/dL)b | 5.5±1.7066 | 5.446±1.53 | 0.93 |
| iPTH (pg/mL)b | 153.659±55.2294 | 140.076±52.79 | 0.51 |
| Pre-dialysis Systolic BP (mmHg)b | 143.33±11.180 | 144.29±11.031 | 0.82 |
| Pre-dialysis Diastolic BP (mmHg)b | 77.33±7.211 | 75.93±9.00 | 0.67 |
| Post-dialysis Systolic BP (mmHg)b | 150±14.142 | 147.86±12.86 | 0.67 |
| Post-dialysis Diastolic BP (mmHg)b | 76±6.928 | 74.36±7.60 | 0.56 |
| Ambulatory BP | |||
| ABPM Systolic BP (mmHg)b | 156.74±22.577 | 154.46±20.82 | 0.78 |
| ABPM Diastolic BP (mmHg)b | 78.39±14.197 | 75.15±10.86 | 0.47 |
| ABPM MAP (mmHg)b | 104.51±17.012 | 102.43±12.77 | 0.69 |
| ABPM SBP >135 mmHg | 9 (100%) | 21 (75%) | 0.05 |
| ABPM DBP | |||
| >85 mmHg | 2 (22.2%) | 5 (17.9%) | 0.77 |
| SDb | 10.5±2.09 | 14.78±3.73 | 0.49 |
| PTEb | 87.92±18.30 | 84.50±12.05 | 0.18 |
| HIb | 643.12±454.54 | 678.59±422.71 | 0.38 |
| Non-Dippera | 7 (77.7%) | 23 (76.6%) | 0.86 |
| ABPM active Systolic BP (mmHg)b | 157.63±23.245 | 154.14±19.561 | 0.66 |
| ABPM active Diastolic BP (mmHg)b | 81.3±13.535 | 75.03±9.539 | 0.13 |
| ABPM active Systolic BP >135 mmHg | 9 (100) | 23 (82.1) | 0.17 |
| ABPM active Diastolic BP >85 mmHg | 2 (22.2) | 4 (14.3) | 0.57 |
| ABPM active MAP (mmHg)b | 107.97±16.553 | 101.88±11.295 | 0.22 |
| ABPM passive Systolic BP (mmHg)b | 157.63±23.245 | 154.14±19.561 | 0.65 |
| ABPM passive Diastolic BP (mmHg)b | 81.3±13.535 | 75.03±9.539 | 0.13 |
| ABPM Passive MAP (mmHg)b | 96.764±5.6582 | 95.477±16.3265 | 0.82 |
| ABPM Passive Systolic BP >135 (n/%) | 8 (88.9) | 21 (75) | 0.38 |
| ABPM Passive Diastolic BP >85 (n/%) | 1 (11.1) | 3 (10.7) | 0.97 |
a Number and percentage, b mean and standard deviation. ABPM - Ambulatory blood pressure monitoring, BP - Blood pressure, iPTH - Intact parathyroid hormone, MAP - Mean arterial pressure, SD - Standard deviation, PTE - Percentage time elevation, HI - Hyperbaric index
Discussion
There is a 10- to 20-fold higher risk for CV events, particularly sudden cardiac death and heart failure, in HD patients than in the general population.[15] HT is an important risk factor for CV disease and mortality in HD patients[16] besides other unique factors such as endothelial dysfunction, arterial stiffness, and increased Fibroblast Growth Factor-23 (FGF23).
The dialysis unit BPs neither predict target organ damage nor all-cause mortality in dialysis patients.[11] Significant variability in an individual's peridialytic BP measurements exists with poor reliability compared with interdialytic BP measurements.[2] This is partly due to the measurement technique[17] because interdialytic self-measured home measurements of BP predict better for left ventricular hypertrophy (LVH) than peridialytic BP measurement.[11] Other factors that dictate inaccurate peridialytic readings include the white coat effect and fluctuations in volume status.[2] The poor reliability of peridialytic BP recordings compared with interdialytic BP recordings by 44-hour ABPM was shown in a meta-analysis.[18] High ambulatory BP is associated closely with increased mortality among HD patients.[8] Meta-analyses of randomized trials established that BP lowering with the use of antihypertensive therapy improves clinical outcomes.[19]
In our observation of 37 elderly dialysis patients by 24-hour ABPM, all were found to have HT (100%), whereas 30 (80%) patients had uncontrolled HT. Nine patients (24.3%) who were normotensive by peridialytic BP measurement were all found to have uncontrolled HT, thus unmasking uncontrolled HT (P = 0.000). Masked HT has been proven to be associated with increased CV risk in the general population.[20] MUCH affects a large proportion of dialysis patients and is associated with increased pulse wave velocity. The prevalence of MUCH ranges from 43% to 75% as measured by ABPM in the interdialytic period. For patients on antihypertensive medications and with elevated predialysis, BPs are more likely to be MUCH.[21] Nondipping of nocturnal BP was observed in the majority of our patients (81%). This is similar to many of the studies showing nondipper status in HD patients in 60% to 80%.[2223] A study from China had shown that more than 90% of patients on dialysis were “nondippers” or “reverse dippers,” with more than half of the patients in the “reverse dipping” group.[24] Most of the patients with uncontrolled HT are nondippers (n = 26, 86.7%), whereas four patients (n = 4, 57.1%) with uncontrolled HT are dippers (P = 0.05). In a prospective study of 57 treated hypertensive HD patients, Amar et al. reported that elevated nocturnal SBP and elevated pulse pressure were independently associated with CV mortality.[8] Fluid retention has been proposed as the major factor contributing to nocturnal HT and nondipping BP patterns in chronic kidney disease (CKD).[25] Nocturnal HT is thought to compensate for sodium retention during the daytime and enhanced pressure natriuresis during the night. Baroreflex dysfunction resulting in impaired BP regulation could contribute to increased BP variability in CKD.[26] Nocturnal hypoxemia that occurs in sleep apnea, which is highly prevalent in dialysis patients, has been associated with nocturnal HT.[27]
An increase in short-term SBP variability was present with advancing CKD stages in a large cohort. This increased SBP variability may be involved in the sharp elevation of CV risk with worsening renal function.[28] We also observed that there is no significant difference in ABPM SD, percentage time elevation (PTE), and hypertension index (HI) between survivors and nonsurvivors.
Our analysis showed that patients with 24-h ABPM uncontrolled systolic HT were also found to have uncontrolled systolic pressures in active (P = 0.0001) and passive periods (P = 0.01) as well as MAP (P = 0.008). CAD occurred more in patients with uncontrolled HT (n = 26, 86.7%, P = 0.05). Also, there was a significant association between uncontrolled 24-hour ABPM SBP and mortality (100%) compared to patients with controlled 24-hour ABPM SBP (P = 0.05). This is consistent with other studies showing increased mortality with elevated interdialytic blood pressure.[8] Gender, diabetic status, dialysis vintage, or adequacy of dialysis had no significant impact on dipping status or mortality in our population. In a study by Agarwal et al., the prevalence of HT in elderly dialysis patients was 86%, and adequate control of HT was found in 30% of patients only. HT was not associated with gender or ethnicity, and risk factors associated with elevated BP included diabetes, old age, and an increased number of antihypertensive in contrast to our experience with no significant association of uncontrolled HT and diabetic status.[9]
Conclusion
The prevalence of uncontrolled HT with blunted circadian rhythm was high among elderly HD patients as detected by ABPM in the interdialytic period. Uncontrolled SBP as well as nocturnal nondipping BP pattern were significantly associated with CAD. MUCH measured by ABPM was not uncommon in patients with normal peridialytic BP. Uncontrolled HT in the interdialytic period had a significant impact on mortality. A prospective randomized-controlled trial with a large sample size will be much more useful.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors wish to thank Ms. Sivasankari Munusamy, our hospital dialysis technologist, for helping to collect the data.
References
- Hypertension and survival in chronic hemodialysis patients—past lessons and future opportunities. Kidney Int. 2005;67:1-13.
- [Google Scholar]
- Age-related blood pressure patterns and blood pressure variability among hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1407-14.
- [Google Scholar]
- A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224-60.
- [Google Scholar]
- The burden of blood pressure-related disease: A neglected priority for global health. Hypertension. 2007;50:991-7.
- [Google Scholar]
- Blood pressure management in hemodialysis: What have we learned? Curr Opin Nephrol Hypertens. 2010;19:561-6.
- [Google Scholar]
- K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1-153.
- [Google Scholar]
- Pro: Ambulatory blood pressure should be used in all patients on hemodialysis. Nephrol Dial Transplant. 2015;30:1432-7.
- [Google Scholar]
- Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Int. 2000;57:2485-91.
- [Google Scholar]
- Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115:291-7.
- [Google Scholar]
- Blood pressure and risk of all-cause mortality in advanced chronic kidney disease and hemodialysis: The chronic renal insufficiency cohort study. Hypertension. 2015;65:93-100.
- [Google Scholar]
- Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006;47:62-8.
- [Google Scholar]
- Toward a definition of masked hypertension and white-coat hypertension among hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2003-8.
- [Google Scholar]
- Advantages of ambulatory blood pressure monitoring in assessing the efficacy of antihypertensive therapy. Cardiol Ther. 2015;4(Suppl 1):5-17.
- [Google Scholar]
- Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395-407.
- [Google Scholar]
- Blood pressure and mortality among hemodialysis patients. Hypertension. 2010;55:762-8.
- [Google Scholar]
- A comparison of standardized versus “usual” blood pressure measurements in hemodialysis patients. Am J Kidney Dis. 2002;39:1226-30.
- [Google Scholar]
- Pre-and post-dialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2006;1:389-98.
- [Google Scholar]
- Cardiovascular protection with antihypertensive drugs in dialysis patients: Systematic review and meta-analysis. Hypertension. 2009;53:860-6.
- [Google Scholar]
- Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: A meta-analysis. J Hypertens. 2007;25:2193-8.
- [Google Scholar]
- Masked uncontrolled hypertension in patients on maintenance hemodialysis. Hypertens Res. 2017;40:819-24.
- [Google Scholar]
- Interdialytic blood pressure obtained by ambulatory blood pressure measurement and left ventricular structure in hypertensive hemodialysis patients. Hemodial Int. 2008;12:322-7.
- [Google Scholar]
- Effects of nutritional parameters on nocturnal blood pressure in patients undergoing hemodialysis. Ren Fail. 2013;35:946-50.
- [Google Scholar]
- Profile of interdialytic ambulatory blood pressure in a cohort of Chinese patients. J Hum Hypertens. 2014;28:677-83.
- [Google Scholar]
- Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004;65:621-5.
- [Google Scholar]
- Differential contribution of afferent and central pathways to the development of baroreflex dysfunction in chronic kidney disease. Hypertension. 2014;63:804-10.
- [Google Scholar]
- Resistant hypertension and obstructive sleep apnea in the setting of kidney disease. J Hypertens. 2012;30:960-6.
- [Google Scholar]
- Blood pressure variability increases with advancing chronic kidney disease stage: A cross-sectional analysis of 16 546 hypertensive patients. J Hypertens. 2018;36:1076-85.
- [Google Scholar]







