Translate this page into:
Karyomegalic Interstitial Nephritis in the Allograft Kidney – A Case Report
Corresponding author: Dr. Anila A. Kurien, Renopath Center for Renal and Urological Pathology, No 27 and 28, VMT Nagar, Kolathur, Chennai - 600 099, Tamil Nadu, India. E-mail: anila_abraham08@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Jansi Prema KS, Mahesh RK, Kurien AA. Karyomegalic Interstitial Nephritis in the Allograft Kidney – A Case Report. Indian J Nephrol. 2024;34:189–90. doi: 10.4103/ijn.ijn_364_22
Abstract
Karyomegalic interstitial nephritis is a rare progressive renal disease. We report a 36-year-old male patient who developed kidney failure due to this condition, underwent kidney transplantation from his sister, and developed the same condition in the graft. Genetic testing of the donor revealed autosomal recessive compound heterozygous mutation of Fanconi anemia–associated nuclease1 (FAN1) gene. Karyomegalic interstitial nephritis is most probably donor derived in our patient. It should not be mislabeled as viral nephropathy.
Keywords
Immunohistochemistry
interstitial nephritis
karyomegaly
kidney biopsy
Introduction
Karyomegalic interstitial nephritis (KIN) is a very rare form of interstitial nephritis with less than 60 cases reported in the native kidney. KIN in renal transplant is extremely rare, with only one case published in the literature. Knowledge of this rare condition is important as it could be misinterpreted as viral nephropathy.
Case Report
A 36-year-old male patient was admitted with sepsis in August 2020. He developed dialysis requiring renal failure. Urinalysis revealed bland urine and subnephrotic proteinuria. Kidney biopsy was performed. Out of the 11 sampled glomeruli, seven (63.6%) were globally sclerotic. Many of the tubules had markedly enlarged, pleomorphic, and hyperchromatic nuclei [Figure 1a and b]. Interstitial fibrosis and tubular atrophy involved 40%–50% of the core. Immunofluorescence study was negative for immunoglobulins, light chains, and complements. Immunohistochemistry for cytomegalovirus and BK polyoma virus was negative. No positive cells were seen on Ki-67 immunostaining. Other secondary conditions were ruled out, and the patient was diagnosed with KIN.
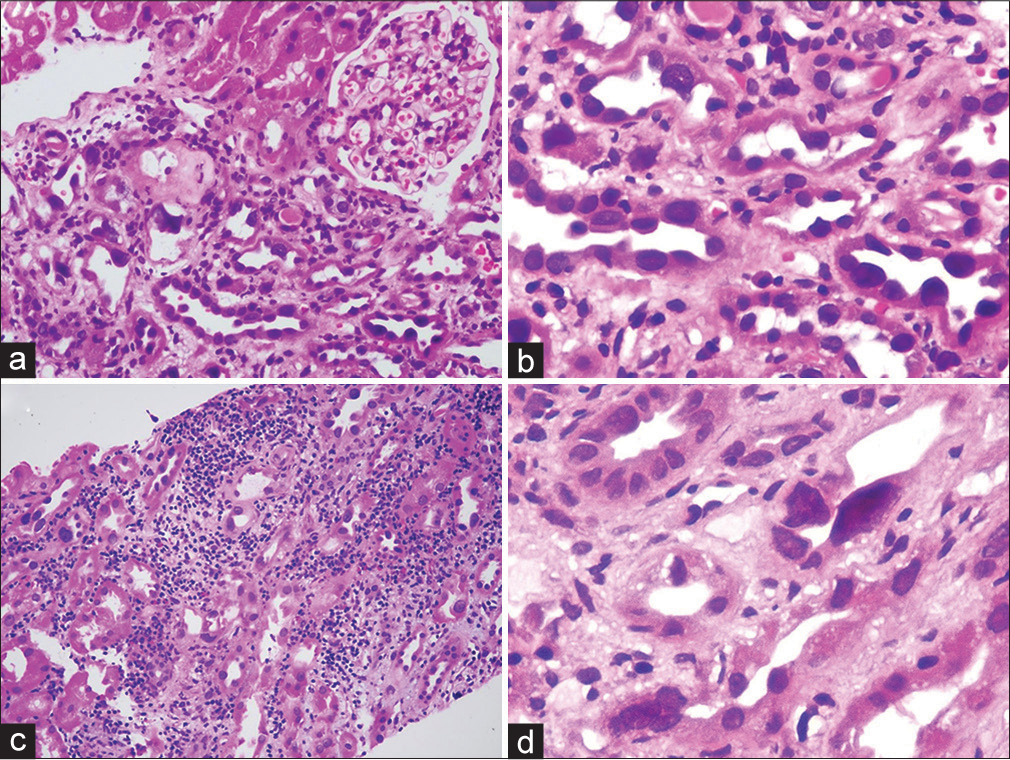
- (a and b) Native kidney biopsy. KIN has focal enlarged tubular epithelial cell nuclei. There is interstitial inflammation and fibrosis along with tubular atrophy (hematoxylin and eosin stain in (a) 20×, in (b) 40×). (c and d) Allograft biopsy. Acute cell-mediated rejection with interstitial inflammation and tubulitis is also seen. Some of the tubular epithelial cells have enlarged nuclei. The biopsy shows large, pleomorphic, and hyperchromatic tubular epithelial cell nuclei with tubular epithelial cell injury (hematoxylin and eosin stain in (c) 20×, in (d) 40×). KIN = karyomegalic interstitial nephritis.
Patient progressed to end-stage renal disease over a period of 14 months. He underwent renal transplantation. Donor was his sister aged 41 years with stable renal functions. He achieved nadir creatinine of 1.7 mg/dl by day 8. He developed acute graft dysfunction. Renal biopsy showed features of acute cell-mediated rejection Banff IA. In addition, many of the tubules had markedly enlarged, pleomorphic, and hyperchromatic nuclei with irregular nuclear membrane [Figure 1c and d]. Immunohistochemistry for BK polyoma virus and cytomegalovirus was negative. Immunohistochemical stain for Ki67 did not stain any of the enlarged nuclei. With pulse steroid therapy, his serum creatinine settled to 1.8 mg/dl.
Genetic testing of the donor done showed autosomal recessive compound heterozygous mutation of Fanconi anemia–associated nuclease1 (FAN1) gene. Patient again developed acute graft dysfunction 16 weeks posttransplant. Repeat biopsy showed features of acute tubular injury and tubular karyomegaly. C4d was negative. Tacrolimus was switched over to everolimus, and his creatinine came down to 2.1 mg/dl.
Discussion
KIN, described first in 1974 is multifactorial disease that could be hereditary, secondary to alkylating agents, heavy metals, ochratoxin exposure, or can be idiopathic.1,2 Mihatsch et al.3 introduced the term KIN in 1979. Since then, less than 60 cases of KIN have been described in native kidney. Only one case of KIN in a renal transplant recipient has been reported so far.4
KIN causes progressive renal failure, leading onto end-stage kidney disease in the early adulthood. Patients usually present with renal failure, mild proteinuria, and hematuria. KIN shows markedly enlarged tubular epithelial cell nuclei. Both proximal and distal convoluted tubules are affected. The degree of interstitial fibrosis and tubular atrophy depends on the stage of the disease.
Mutations in FAN1 gene have been implicated in KIN.5 FAN1 plays an important role in DNA interstrand crosslink repair and controls ploidy in renal tubular epithelial cells.
In our study, the donor had compound heterozygous mutation involving FAN1 gene. It was proposed that persons with heterozygous mutations in FAN1 are asymptomatic. It is possible that the index patient could have homozygous FAN1 mutation that has resulted in progressive kidney damage.
Ravindran et al.4 reported KIN 4 months posttransplant; in our case, karyomegalic tubular epithelial cells were noted 16 days posttransplant. It is more likely to be donor derived since donor genetic studies revealed FAN1 mutation.
The most important differential diagnosis of KIN in a renal allograft biopsy is viral nephropathy.4 Though enlarged nuclei are seen in both the conditions, intranuclear inclusions are not identified in KIN. SV-40 and cytomegalovirus immunohistochemistry is very specific and helps in the confirmation of viral nephropathy.
One should also differentiate KIN from reactive tubular epithelial changes associated with acute cell-mediated rejection. Reactive nuclei are seen in areas of active inflammation, associated with tubulitis; nuclei are not as enlarged as in KIN and they resolve on treatment of underlying rejection.
There is no specific treatment for KIN. There is limited data regarding the prognosis of post transplant KIN. Ravindran et al.4 reported stable graft function 24.7 months post transplant. Our patient and donor have stable renal function 9 months post transplant.
One should suspect KIN when a young individual in the second or third decade presents with progressive renal failure. It is imperative to differentiate KIN from viral nephropathy for correct treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Karyomegalic interstitial nephritis. Medicine (Baltimore). 2016;95:3349. doi: 10.1097/MD.0000000000003349
- [CrossRef] [PubMed] [Google Scholar]
- Extreme dysplasla in renal epithelium of a young woman dying from hepatocarcinoma. J Pathol. 1974;113:147-50.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic karyomegaly associated with chronic interstitial nephritis. A new disease entity? Clin Nephrol. 1979;12:54-62.
- [Google Scholar]
- Karyomegalic interstitial nephritis in a renal allograft. Am J Transplant. 2019;19:285-90.
- [CrossRef] [PubMed] [Google Scholar]
- FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat Genet. 2012;44:910-5.
- [CrossRef] [PubMed] [Google Scholar]







