Translate this page into:
Maternal, Fetal, and Kidney Outcomes of Viable Pregnancies in Women with Underlying Kidney Disease: Experience from a Single Tertiary Care Center in India
Corresponding author: Selvin Sundar Raj Mani, Department of Nephrology, Christian Medical College, Vellore, Tamil Nadu, India. E-mail: selvinsr@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Johny J, Mani SSR, Alam R, Jose N, Lalwani M, Eapen JJ, Thomas A, et al. Maternal, Fetal, and Kidney Outcomes of Viable Pregnancies in Women with Underlying Kidney Disease: Experience from a Single Tertiary Care Center in India. Indian J Nephrol. doi: 10.25259/IJN_507_2024
Abstract
Background
There is a dearth of data from resource-limited settings on pregnancy outcomes in women with kidney disease. We investigated the maternal, fetal, and renal outcomes of pregnancies amongst women with kidney disease and compared these outcomes with normal pregnancy outcomes.
Materials and Methods
This was a single-center retrospective observational study. The maternal, fetal, and renal outcomes of women with kidney dysfunction who delivered at the study center after at least 22 weeks of gestation (viable pregnancy) between January 2012 and December 2021 were analyzed. The study results were also compared with the outcomes of normal pregnancy.
Results
Two hundred and one deliveries were included in the study, constituting 0.14% of all deliveries during the study period. Lupus nephritis (39.3%) and Immunoglobulin A nephropathy (11.4%) were the most common underlying kidney diseases. The mean (±SD) gestational age at delivery was 34.9 (±3.7) weeks. A higher cesarean section rates (45.3%) were observed in these high-risk pregnancies. Low birth weight was observed in 51.7% of all deliveries. Renal outcome regarding proteinuria and estimated glomerular filtration rate was excellent. Renal transplant patients had unfavorable outcomes. More than 45% of patients had term deliveries with normal birth weight.
Conclusion
Satisfactory maternal, fetal, and renal outcomes can be achieved with proper pre-pregnancy counseling and multidisciplinary care in women with kidney diseases, even in resource-limited settings.
Keywords
Hypertension
Kidney disease
Kidney transplantation
Low birth weight
Pregnancy
Introduction
The management of pregnancy in a woman with underlying kidney disease is challenging. Globally, almost 3% of pregnant women are affected by kidney disease.1 With the advances in nephrology and obstetric care, the outcome of pregnancies in such women, including those on dialysis has improved. There are no data from developing countries on the outcomes of such pregnancies. Our center, a large tertiary educational referral center in South India, handles high-risk pregnancies. This study aims to derive data on the outcome of pregnancies in women with preexisting kidney disease, which may help physicians provide effective pre-pregnancy counseling and management as well as improve the maternal, perinatal, and renal outcomes of pregnancies with kidney disease.
Materials and Methods
This study was approved by the Institutional Review Board (IRB number 14741 dated 29.06.2022). This was a single-center retrospective observational study conducted in the Department of Nephrology and Obstetrics at the Christian Medical College Vellore, one of India’s largest healthcare centers. All consecutive viable pregnancies (more than 22 weeks of gestational age) in women with underlying kidney disease, who delivered at our center between January 2012 and December 2021 were included. Only those women diagnosed with kidney disease before pregnancy or in the first trimester were included. The estimated glomerular filtration rate (eGFR) was calculated using the chronic kidney disease epidemiology collaboration (CKD-EPI) formula2 and the kidney disease improving global outcomes (KDIGO) criteria was used for defining chronic kidney disease (CKD).3
Viable pregnancy was defined according to the American College of Gynaecology (ACOG) criteria as pregnancy after 22 weeks of gestation. Gestational hypertension was defined as a new onset of hypertension (systolic blood pressure >140 mm Hg and diastolic blood pressure >90 mmHg on two occasions at least four to six hours apart). Preeclampsia and eclampsia were defined as per the ACOG criteria.4
The World Health Organization (WHO) criteria were used to define preterm birth as live birth before 37 weeks of gestation, while low birth weight was defined as birth weight of less than 2500 g at the time of birth, regardless of the gestational age.
The data on baseline characteristics, demographic details, and obstetric details were obtained from a prospectively maintained pregnancy data registry at the Department of Obstetrics and Gynaecology at our center. The data on kidney function, native kidney disease, clinical characteristics, treatment details, and follow-up were collected from the electronic medical records; these records were reviewed for follow-up till December 2022.
Statistical analysis
Baseline clinical characteristics along with kidney, maternal, and fetal outcomes of pregnancies were entered in the Epidata version 4.6.0.6. Analysis was done using SPSS software version 25. Baseline characteristics were reported as mean ± standard deviation (SD) for normally distributed quantitative variables and median (IQR) for skewed variables. Outcomes of pregnancies were compared between the groups based on the underlying of kidney disease, eGFR, and degree of proteinuria. Difference in outcomes in the subgroups was calculated using the chi-square test or the one-way analysis of variance (ANOVA) as appropriate. The study results were also compared with the outcomes of all pregnancies at our center during the same period and with the national data.
Results
Data on 201 viable pregnancies in women with underlying kidney disease were collected, which constituted 0.14% of a total of 138,110 deliveries during the study period. The mean age (±SD) at conception was 27.4 (±4.6) years, and 44.3% were primigravida. The most common clinical kidney syndrome at the initial presentation was CKD (34.8%), followed by nephrotic syndrome (32.8%). Hypertension at the time of conception was present in 23.9% of the women.
Lupus nephritis (LN), with or without antiphospholipid antibody (APLA) syndrome (39.3%), and immunoglobulin A (IgA) nephropathy (11.4%) were the common underlying kidney diseases among our patients. Renal disease was detected first during the first trimester in 40 (19.9%) pregnancies. Other baseline characteristics are summarized in Table 1.
| Variables | N (%) or mean ± SD or Median IQR |
|---|---|
| Demographic characteristics | |
| Age at conception | 27.4 ± 4.6 |
| Primigravida | 89 (44.3) |
| Past obstetric complications | |
| Past abortions and still births | 69 (34.3) and 26 (12.9) |
| Gestational hypertension | 27 (13.4) |
| Preeclampsia/eclampsia/HELLP syndrome | 19 (9.5)/2 (1)/3 (1.5) |
| Preterm delivery | 39 (19.4) |
| Fetal distress | 13 (6.5) |
| Native kidney disease | |
| Lupus nephritis (LN)/LN with APLA | 70 (34.8)/9 (4.5) |
| IgA nephropathy | 23 (11.4) |
| Focal segmental glomerulosclerosis | 16 (8) |
| Minimal change disease | 9 (4.5) |
| Diabetic kidney disease | 7 (3.5) |
| Kidney transplant recipients | 6 (2.9) |
| Membranous nephropathy | 5 (2.5) |
| Alport/ADPKD | 2 (1) each |
| Others† | 52 (25.9) |
| Maternal comorbidities | |
| Maternal diabetes mellitus | 10 (5) |
| Hypertension | 48 (23.9) |
| Anemia | 88 (43.8) |
| Hypothyroidism | 30 (14.9) |
| Family history of kidney disease | 4 (2) |
| Past acute kidney injury | 3 (1.5) |
| Teratogenic drug intake | 25 (12.4) |
| Current pregnancy | |
| Anemia | 88 (43.8) |
| Gestational diabetes mellitus | 28 (13.9) |
| Renal disease detected first during the first trimester | 40 (19.9) |
| Duration of kidney disease in years before pregnancy | 6 (3,12) |
| Clinical kidney syndrome | |
| Nephrotic syndrome | 66 (32.8) |
| Nephritic syndrome | 64 (31.8) |
| Rapidly progressive renal failure | 1 (0.5) |
| CKD | 70 (34.8) |
|
Kidney replacement therapy (KRT) at conception KRT mode |
6 (2.9) Renal transplant (100%) |
APLA: Antiphospholipid antibody syndrome, ADPKD: Autosomal dominant polycystic kidney disease, CKD: Chronic kidney disease, HELLP: Hemolysis, elevated liver enzymes, and low platelet counts syndrome, †: Obstructive uropathy, C3 glomerulonephritis, renovascular disease, chronic interstitial nephritis. SD: Standard deviation, IQR: Interquartile range
Maternal outcomes
Gestational hypertension, preeclampsia, and eclampsia occurred in 20.4%, 21.9%, and 1.5% of pregnancies, respectively. Preterm delivery was observed in 37.8% of pregnancies. A large portion of the deliveries were induced (65.2%). The mean (±SD) gestational age at delivery was 34.9 (±3.7) weeks, and cesarean and instrumental deliveries accounted for 45.3% and 13.4%, respectively. Hypertensives disorders of pregnancy (25.3%) and fetal distress (23.1%) were the most common indications for cesarean deliveries [Table 2]. One (0.5%) patient died in the immediate postpartum due to sepsis and disseminated intravascular coagulation following severe post-partum hemorrhage.
| Outcome | N (%) |
|---|---|
| Maternal outcomes | |
| Gestational hypertension | 41 (20.4) |
| Preeclampsia | 44 (21.9) |
| HELLP syndrome | 3 (1.5) |
| Eclampsia | 3 (1.5) |
| Induced labor | 131 (65.2) |
| Puerperal sepsis | 15 (7.5) |
| Mode of delivery | |
| Normal | 83 (41.3) |
| Instrumental | 27 (13.4) |
| LSCS | 91 (45.3) |
| LSCS indication | |
| Fetal distress | 21 (23.0) |
| Preeclampsia/HELLP | 23 (25.3) |
| Previous LSCS | 18 (19.8) |
| Non-progression of labor | 24 (26.4) |
| Unfavorable fetal position | 5 (5.5) |
| Fetal outcomes | |
| Gestational age at delivery (weeks) | 34.95 (3.7) |
| Fetal distress | 31 (15.4) |
| Mean birth weight (kg) | 2.2 (0.75) |
| Low birth weight | 104 (51.7) |
| Perinatal asphyxia | 15 (7.5) |
| Neonatal ICU care | 22 (10.9) |
| Delivery outcome | |
| Live with normal birth weight | 95 (47.3) |
| Live with low birth weight | 89 (44.3) |
| Still birth | 9 (4.5) |
| Intrauterine death | 5 (2.5) |
| Live with large gestational age | 1 (0.5) |
| Neonatal death | 2 (1) |
| Proteinuria (mg/day) | |
| At conception | 110 (80,325) |
| First trimester | 150 (80,494) |
| Second trimester | 187 (90,753) |
| Third trimester | 295 (120,1590) |
| Postpartum | 300 (100,1350) |
| Last follow-up | 205 (110,1068) |
| Systolic blood pressure (mm Hg) | |
| At conception | 116 (13.7) |
| First trimester | 114 (14.1) |
| Second trimester | 115 (13.4) |
| Third trimester | 121 (15.9) |
| Postpartum | 119 (12.9) |
| Last follow-up | 120 (16.3) |
| eGFR ml/min/sq.m | Mean (SD) |
| At conception | 101.2 (33.9) |
| First trimester | 108.9 (37.0) |
| Second trimester | 112.5 (37.1) |
| Third trimester | 106.7 (39.5) |
| Postpartum | 101.3 (39.6) |
| Last follow-up | 96.7 (43.0) |
| Total duration of follow-up (months) | 32 (7,72.5) |
| Dialysis dependent at follow-up | 7 (3.5) |
eGFR: Estimated GFR, HELLP: Hemolysis, elevated liver enzymes, and low platelet counts syndrome, ICU: Intensive care unit, LSCS: Lower segment cesarean section.
Fetal outcomes
The mean (±SD) birth weight was 2.2 kg (±0.75) with perinatal asphyxia occurring in 15 (7.5%) neonates. Twenty-two (10.9%) neonates required intensive care unit (ICU) care. Eighty-nine (44.3%) live births had low birth weight. Deaths (intrauterine deaths, still birth, or neonatal deaths) were seen in 16 (7.9%) deliveries [Table 2].
Kidney outcomes
The mean eGFR at the time of conception was 101.2 (±33.9) mL/min/1.73 m2. There was an increase in eGFR of 6.9% and 11.1% in the first and second trimesters, respectively, as compared to the pre-pregnancy level, indicating less than the expected increase in eGFR in normal pregnancy [Table 2]. The eGFR at the last follow-up was comparable to the pre-pregnancy level [Figure 1]. Seven patients (3.5%) required kidney replacement therapy at a median time of 32 (7,72.5) months of follow-up. The median (IQR) proteinuria before conception was 110.5 mg/day (80,325). There was a progressive increase in proteinuria during pregnancy, reaching a maximum in the postpartum period, which then decreased to a median (IQR) proteinuria of 205 mg/day (110,1068) at the last follow-up [Figure 2]. The physiologic fall in blood pressure, observed in normal pregnancy, was not observed in these patients.
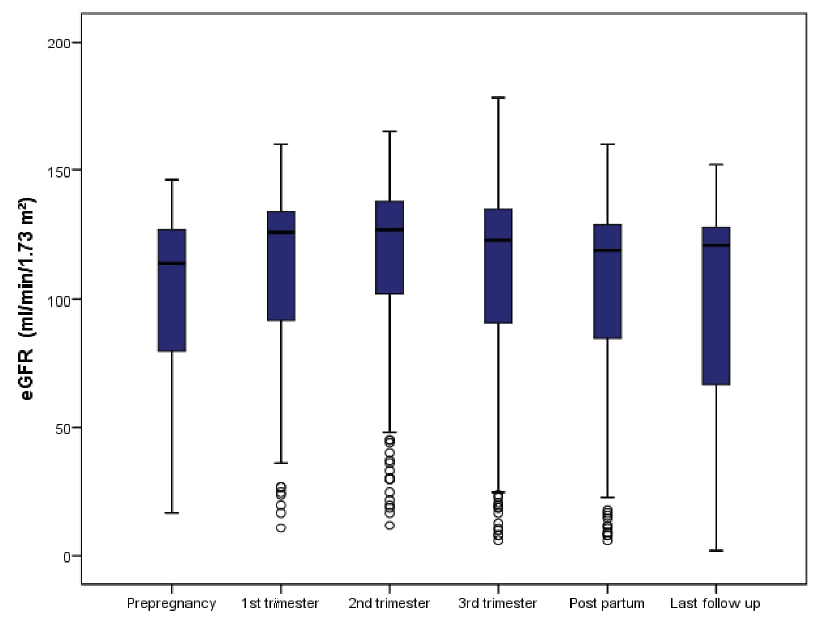
- Boxplot showing the renal function in terms of median eGFR before pregnancy, at first trimester, second trimester, third trimester, postpartum and at the last follow up. eGFR: estimated glomerular filtration rate.
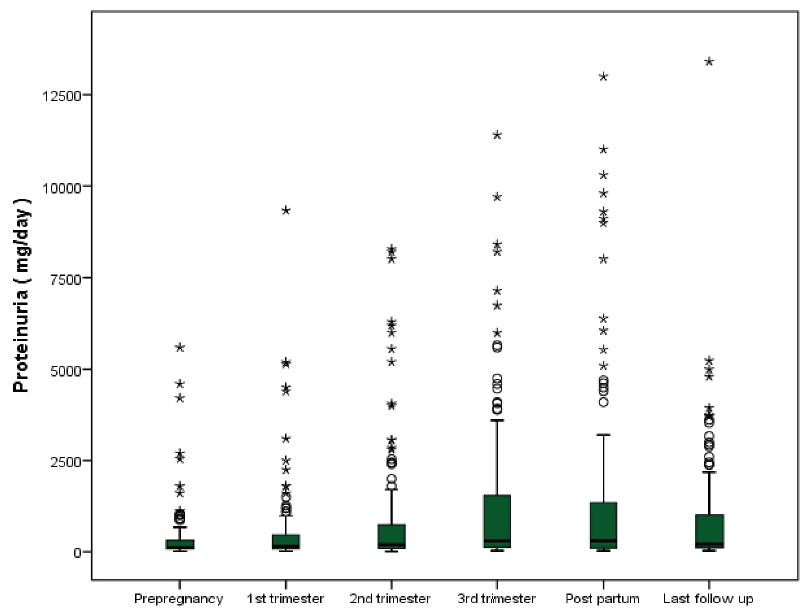
- Boxplot showing the renal function in terms of median proteinuria before pregnancy, at first trimester, second trimester, third trimester, postpartum and at the last follow up.
Comparison of data among subgroups
No statistically significant differences were observed in the fetal and maternal outcomes between various native kidney subgroups. There were consistently high LSCS rates (>40%), preterm deliveries (>30%) and low birth weight rates (>30%) in all the subgroups [Table 3]. As the degree of proteinuria increased, there was a statistically significant increase in the rates of complications [Table 3]. Subgroup analysis of eGFR showed a statistically significant increase in preeclampsia and preterm delivery as the eGFR level decreased [Table 3].
| Variables | N (%) | N (%) | N (%) | p value |
|---|---|---|---|---|
| Native kidney disease | LN, N = 79 | IgAN, N = 23 | NS, N = 30 | |
| Gestational hypertension | 14 (17.7) | 5 (21.7) | 3 (10) | 0.49 |
| Preeclampsia | 13 (16.5) | 5 (21.7) | 6 (20) | 0.77 |
| Preterm delivery | 31 (39.2) | 7 (30.4) | 11 (36.7) | 0.74 |
| LSCS deliveries | 39 (49.4) | 11 (47.8) | 13 (43.3) | 0.85 |
| Low birth weight | 39 (49.4) | 8 (34.8) | 14 (46.7) | 0.49 |
| 24-hour urine protein (mg) | <500, N = 120 | 500–1500, N = 34 | >1500, N = 47 | |
| Gestational hypertension | 14 (11.7) | 10 (29.4) | 17 (36.2) | 0.001 |
| Preeclampsia | 12 (10) | 12 (35.3) | 20 (42.6) | <0.001 |
| Preterm delivery | 30 (25) | 19 (55.9) | 27 (57.4) | <0.001 |
| LSCS | 55 (45.8) | 19 (55.9) | 17 (36.2) | 0.21 |
| Low birth weight | 54 (45) | 20 (58.8) | 30 (63.8) | 0.005 |
| eGFR (mL/min/1.73 sq.m) | <60 (N = 32) | 60–89 (N = 22) | >90 (N = 147) | |
| Gestational hypertension | 9 (28.1) | 5 (22.7) | 27 (18.4) | 0.46 |
| Preeclampsia | 13 (40.6) | 5 (22.7) | 26 (17.7) | 0.02 |
| Preterm delivery | 18 (56.3) | 9 (40.9) | 49 (33.3) | 0.05 |
| LSCS | 15 (46.9) | 12 (54.5) | 64 (43.5) | 0.64 |
| Low birth weight | 14 (43.8) | 10 (45.5) | 69 (46.9) | 0.06 |
eGFR: estimated GFR, IgAN: IgA nephropathy, LN: Lupus nephritis, LSCS: lower segment cesarean section, NS: Nephrotic syndrome due to minimal change disease, Focal segmental glomerulosclerosis and membranous nephropathy. Bold values indicate statistically significant values.
The prevalence of hypertensive disorders in pregnancy with renal dysfunction was more than thrice that in pregnant women without renal dysfunction. The mean birth weight was lower and LSCS rates were higher in the study population. Low birth weight deliveries in the renal dysfunction group occurred more than twice that in those without renal dysfunction [Table 4].
| Outcomes | Present study | All deliveries at the study site during the study period | NFHS 2019–2021 |
|---|---|---|---|
| Gestational hypertension | 20.4% | 6.1% | |
| Preeclampsia | 21.9% | 4.9% | |
| Gestational age in weeks at delivery | 34.95 ± 3.6 | 37.96 ± 0.58 | |
| Preterm delivery | 37.8% | 13.6% | 12.00% |
| Lower segment cesarean section | 45.3% | 32.7% | 21.5% |
| Low birth weight deliveries | 44.3% | 19.9% | 18.2% |
| Mean birth weight (kg) | 2.20 ± 0.75 | 2.85 ± 0.58 |
NFHS: National family health survey (India)
As compared to the national data from the National Family Health Survey 5 (2019–2021),5 women with renal dysfunction had a significant increase in the rates of preterm deliveries, LSCS, and low birth weight, highlighting the risk associated with such pregnancies [Table 4].
Renal transplant recipients outcome data
Six patients were kidney transplant recipients, with a mean (±SD) age of 21.33 (±6.43) years. The transplant vintage at delivery was between 4 and 17 years. Five (83.3%) patients had a planned pregnancy, and IMS (immunosuppression) was changed to non-teratogenic drugs at least three months before conception. All except one had creatinine less than 1.5 mg/dL and proteinuria of less than 500 mg/day at the time of conception. The sixth patient had proteinuria 1.2 gm/day and creatinine 2.4 mg/dL. The renal biopsy in the second trimester showed chronic IgA nephropathy (IgAN). She delivered at 31 weeks’ gestation by LSCS, the indication being severe preeclampsia, and the baby’s birth weight was 1220 gm [Supplemental Table 1]. This patient, on follow-up after one year, had worsening proteinuria of 8 gm/day with an eGFR of 14.2 mL/min/1.73m2. Of the other five pregnancies, only one patient had term delivery of a fetus with appropriate birth weight of 3000 gms, and the other four had preterm deliveries. One pregnancy resulted in intrauterine death, while another had twins with an early neonatal death of one of the twins. At the time of the last follow-up, one patient had a failing allograft, one had graft dysfunction with a GFR of 61 mL/min/1.73 m2, and one had become dialysis-dependent five years after delivery [Supplemental Table 1].
Discussion
This study demonstrates that kidney disease in a pregnant woman is associated with worse maternal and fetal outcomes compared to those without renal dysfunction. This is the first study of such a large sample size from a developing country. The prevalence of kidney disease in pregnant women in our study was 0.14%, although the estimated prevalence of kidney disease among women of childbearing age is 3%. This may be probably due to the hesitancy among the young women and the treating physicians to continue with such high-risk pregnancies and because we have included only viable pregnancies in our study.6
The physiological increase in the GFR and fall in blood pressure was less than expected in a normal pregnancy.7 About a quarter of patients were known to be hypertensive at conception, similar to the previous studies, which reported a range between 9-40%.8
Our study had LN as the most common underlying kidney disease unlike previous studies which reported IgAN as the most common kidney disease in pregnant women.8,9 Both these kidney diseases constituted more than 50% of the underlying kidney diseases reflecting the distribution of these diseases in childbearing age.
Gestational age at delivery and birthweight were lesser than the previous studies from the developed world. Our study also found higher proportions of hypertensive disorders and LSCS rates. Neonatal ICU care and infants death were more common in our study.10-12 However, maternal death was much less in the present study. Racial and socioeconomic factors and difference in the referral practices would have resulted in these outcomes.
Although some studies have indicated that the type of glomerular disease affects the pregnancy outcome, our study failed to demonstrate any significant difference in the outcome between different glomerular diseases.13 This finding is consistent with the results of recent studies.8,14,15 However, the largest subgroup (LN) had more than twice the rates of hypertensive disease, preterm births, low birth weight deliveries and higher LSCS deliveries compared to the normal pregnancies during the same period. The figures were higher than that found in similar studies.16,17
The second largest subgroup (IgA nephropathy) also had worse maternal and fetal outcomes.14
An important finding was that as the level of proteinuria increased and renal function worsened in terms of estimated glomerular filtration rate, there was a statistically significant increase in the adverse maternal and fetal outcomes irrespective of the underlying cause – a finding consistently seen in the previous studies.10-12,18 It highlights the fact that women with mild kidney dysfunction and lesser proteinuria may have favorable pregnancy outcomes.19
A meta-analysis by Zhang et al.12 and other studies on kidney disease in pregnancy have shown that long term renal outcome is favorable.10,20 Our study also showed stable kidney function following pregnancy as evidenced by the stable GFR and proteinuria on long term follow up. This is an important finding as the main concern for the nephrologist is the possibility of deleterious effect of pregnancy on the long-term kidney function. However, our cohort had lesser women with eGFR less than 30 ml/minute/1.73 sq.m and nephrotic range proteinuria which needs further evaluation.
Our study also has shown that women on renin angiotensin aldosterone (RAAS) inhibitors at the time of conception did not have any fetal abnormalities similar to the finding shown in a study by Bilal et al.21 This finding is important as some nephrologists may advise termination of pregnancy in such women rather than withholding the drug and continuing the pregnancy.
Our study had only 6 kidney transplant patients. Although reliable conclusions cannot be drawn because of smaller sample size, our study showed worse pregnancy outcome as shown by Deshpande et al.22 Contrary to the findings of the metanalysis by Marleen C. van Buren and a prospective study by Aivazoglou et al. our patients had poor kidney outcome with half of the patients having a GFR of less than 20 ml/min/1.73m2 on long term follow up.23,24
Successful pregnancies in women on dialysis are being reported from worldwide.25 However our study did not have any patients on dialysis reflecting low fertility rates and the hesitancy of the patient and physician.
Our study had a few limitations: It was a single-center study. We used the CKD-EPI equation to estimate GFR, which had not yet been validated in pregnancy. There were no women on dialysis. Despite the limitations mentioned above, it was the largest study reported from a developing country like India.
In conclusion, women with underlying kidney disease, including those with reasonably good kidney function at conception had worse pregnancy outcomes compared to the normal pregnancy. However, long-term kidney outcomes were favorable.
Acknowledgments
We acknowledge the contribution of Mrs. Grace Rebecca, biostatistician for the statistical analysis of the results and Dr. Pippa Deodar for the proof reading of the manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- Pregnancy with renal disease: Present scenario in tertiary care institute in northern India. J Obstet Gynaecol India.. 2022;72(3):201-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A new equation to estimate glomerular filtration rate. Ann Intern Med.. 2009;150(9):604-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney International.. 2005;67(6):2089-100.
- [CrossRef] [PubMed] [Google Scholar]
- Gestational hypertension and preeclampsia: ACOG practice bulletin, Number 222. Obstetrics & Gynecology.. 2020;135(6):e237-60.
- [PubMed] [Google Scholar]
- Release of NFHS-5 (2019-21) - Compendium of factsheets | Ministry of health and family welfare | GOI [Internet]. [cited 2024 Feb 20]. Available from: https://main.mohfw.gov.in/?q=basicpage-14
- Renal disease in pregnancy: Fetal, neonatal and long-term outcomes. Best Pract Res Clin Obstet Gynaecol.. 2019;57:60-76.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease and pregnancy. Semin Nephrol.. 2017;37(4):337-46.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy and glomerular disease: A systematic review of the literature with management guidelines. Clin J Am Soc Nephrol.. 2017;12(11):1862-72.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pregnancy outcomes in patients with glomerular disease attending a single academi center in north carolina. Am J Nephrol.. 2017;45(5):442-51.
- [CrossRef] [PubMed] [Google Scholar]
- Kidney disease and maternal and fetal outcomes in pregnancy. Am J Kidney Dis.. 2015;66(1):55-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pregnancy and chronic kidney disease: a challenge in all CKD stages. Clin J Am Soc Nephrol.. 2010;5(5):844-55.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A Systematic review and meta-Analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol.. 2015;10(11):1964-78.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A Systematic review and meta-analysis of kidney and pregnancy outcomes in IgA nephropathy. Am J Nephrol.. 2016;44(3):187-93.
- [CrossRef] [PubMed] [Google Scholar]
- Kidney outcomes and risk factors for nephritis (Flare/De Novo) in a multiethnic cohort of pregnant patients with lupus. Clin J Am Soc Nephrol.. 2017;12(6):940-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Fetal outcome and recommendations of pregnancies in lupus nephritis in the 21st century. A prospective multicenter study. J Autoimmun. 2016;74:6-12.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol.. 2010;5(11):2060-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pregnancy in women with immunoglobulin A nephropathy: are obstetrical complications associated with renal prognosis? Nephrol Dial Transplant.. 2018;33(3):459-65.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features and prognosis of pregnancy-related renal damage and pregnancy after chronic kidney disease. BMC Pregnancy Childbirth.. 2023;23:619.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy and progression of IgA nephropathy: results of an Italian multicenter study. Am J Kidney Dis.. 2010;56(3):506-12.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal and perinatal outcomes associated with the use of renin-angiotensin system (RAS) blockers for chronic hypertension in early pregnancy. Pregnancy Hypertens.. 2018;14:156-61.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant.. 2011;11(11):2388-404.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term graft survival and graft function following pregnancy in kidney transplant recipients: A systematic review and meta-analysis. Transplantation.. 2020;104(8):1675-85.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pregnancy after renal transplantation: an evaluation of the graft function. Eur J Obstet Gynecol Reprod Biol.. 2011;155(2):129-31.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy in advanced chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens.. 2015;24(3):252-9.
- [CrossRef] [PubMed] [Google Scholar]








