Translate this page into:
MHC Class I Related Chain A (MICA) Antibodies - A Potential Cause of Renal Allograft Rejection
Address for correspondence: Prof. Narinder Mehra, National Chair and Former Dean, All India Institute of Medical Sciences, Ansari Nagar, New Delhi - 110 029, India. E-mail: narin.mehra@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
MHC class I related chain A (MICA) antibodies, especially those directed against the donor in absence of donor-specific anti-HLA antibodies have been reported to be possibly associated with renal allograft rejection in sensitized recipients. We are the first ones to present a case series of five patients who underwent primary live related donor renal transplantation in non-sensitized recipients either in the presence of donor-specific MICA antibodies (MICA-DSA) or developed de novo. Four of them presented characteristics of either accelerated, acute or chronic antibody-mediated rejection (AMR) attributable to the presence of MICA DSA. This case series emphasizes that AMR due to MICA-DSA is amenable to treatment with conventional regimens for treatment of AMR and there is a need for screening of MICA antibodies especially those directed against the donor on case to case basis.
Keywords
Antibody mediated rejection
donor specific antibodies
MICA
renal transplantation
Introduction
MHC class I related chain A (MICA) gene is located in the MHC class I region centromeric to the HLA-B locus on chromosome 6. It is the most polymorphic non-HLA antigenic target, with 223 alleles already reported at present while new alleles are continuously being identified (http://hla.alleles.org/nomenclature/stats.html, updated Jul 2020). Unlike the ubiquitous expression of HLA class I molecules, MICA have limited tissue distribution being expressed constitutively on endothelial cells, epithelial cells especially in the gastrointestinal tract, fibroblasts, monocytes and dendritic cells.[1] They are not expressed on resting T & B lymphocytes; however, their expression can be induced on activated lymphocytes.
The first concrete study suggesting a possible association of MICA antibodies with renal allograft outcome was published in 2007.[2] Since then, several investigators have highlighted the impact of MICA antibodies on graft outcome albeit very few have focussed on donor specificity of these antibodies.[3456] MICA antibodies especially the donor-specific ones have been found to be associated with hyperacute, accelerated acute, acute and chronic rejection and there are few case reports to that effect both in adult and the paediatric age groups.[789]
We present here five cases who underwent primary live related donor renal transplantation either in the presence of donor-specific MICA antibodies (MICA-DSA) or developed de novo. MICA genotyping of the recipients and donors in all five cases was accomplished using the commercially available LABType SSO (One Lambda Inc. USA) kit. MICA and HLA antibodies were determined by single antigen bead (SAB) assay (One Lambda Inc USA) on Luminex platform.
Case Reports
Case 1
A 25-year-old male patient was transplanted against a negative complement-dependent cytotoxicity (CDC) crossmatch with no evidence of panel reactive antibodies (PRA). The donor was his HLA-haploidentical mother with a total mismatch grade of 2/6 in the HLA-A, -B and -DR loci. The patient was maintained on a triple-drug immunosuppressant (TDI) regimen that included Tacrolimus, MMF and Wysolone. Posttransplant follow up showed a rise in creatinine to 4.6 mg/dl on postoperative day (POD) 5. At this point of time, graft biopsy was done which revealed C4d negative antibody-mediated rejection (AMR). Retrospective analysis on the pretransplant serum as well as that obtained at the time of biopsy revealed the presence of DSA against MICA *009 allele with MFI value 13907 and 9945 respectively. Patient was treated with plasmapheresis, IVIG and Bortezomib to which he responded well and by day 30, his creatinine settled down to 1.6 with disappearance of anti-MICA antibody in the circulation [Figure 1]. HLA antibodies by SAB assay were not detected in the patient at any point of time. The patient was followed up to 16 months post-transplant and during this period, no further rejection episode was noted and he remained asymptomatic with creatinine of 1.6 mg/dl.
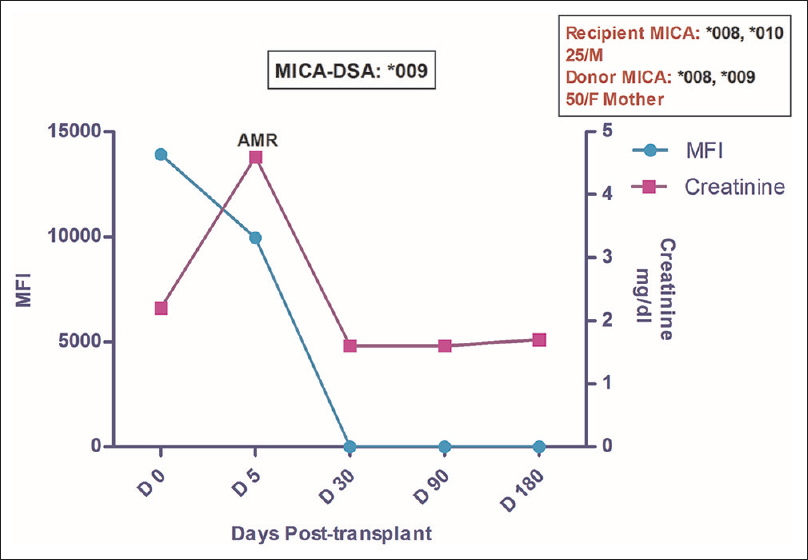
- Longitudinal DSA analysis of case 1: Patient had DSA against MICA *009 at pre transplantation with MFI of 13907. No HLA-DSA was detected. Patient developed AMR (C4d–ve) on day 5 and MFI at that point of time fell down to 9945 with ↑ in creatinine to 4.6 from pre-transplant level of 2.2. The patient was treated for AMR which led to disappearance of MICA antibodies post treatment while the creatinine settled to a baseline value of 1.6. The patient was followed up to 16 months post-transplant and during this period no further rejection episode was observed
Case 2
A 31-year-old female was transplanted against a negative CDC crossmatch and PRA of 0%. Mother was the HLA haploidentical donor. The patient was maintained on TDI. Postoperative creatinine fell to 2.8 mg/dl on POD 7, which further increased to 3 mg/dl on POD 12. At this point of time, graft biopsy was done which revealed an evidence of C4d + AMR. DSA analysis by SAB assay on serum sample obtained at the time of biopsy as well as on the pretransplant sample showed absence of HLA-DSA in the circulation. However, MICA-DSA against MICA *027 allele was present in both sera samples with MFI of 13789 and 12945 respectively. The patient was treated with plasmapheresis, IVIG and Bortezomib to which she responded well and by day 30, her creatinine settled down to 1.8 mg/dl which further dropped to the baseline value of 1.6 mg/dl and neither HLA nor MICA antibody was detected in the circulation [Figure 2]. Posttransplant period has since been uneventful with no evidence of any circulating antibody.
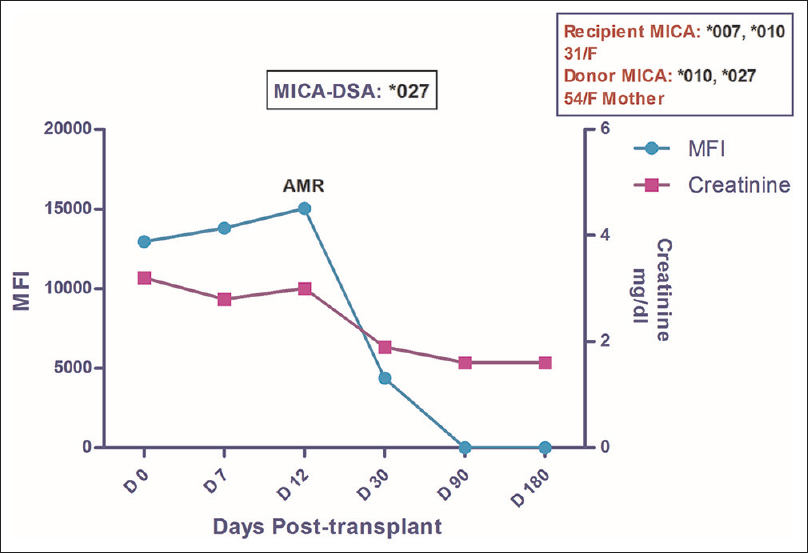
- Longitudinal DSA analysis of Case 2: The patient had DSA against MICA*027 at pre transplantation with MFI of 12945. She developed AMR on POD 12, was treated successfully for the same which led to disappearance of MICA antibodies by day 90 while the creatinine settled down to a baseline value of 1.6. No further rejection episode was observed till the post-transplant follow-up period of 23 months
Case 3
A 30 yrs old male patient underwent renal transplantation with wife as the donor and a 3/6 HLA mismatch status. Pretransplant Luminex PRA was 0% and CDC crossmatch was negative. He was given Basiliximab induction as per protocol and was maintained on TDI. His Creatinine remained 3.2 mg/dl on POD 7. Detailed evaluation including biopsy at this point of time revealed that patient had acute tubular necrosis (ATN). He was managed for ATN and subsequently his creatinine settled to a 1.6 mg/dl by POD 30 and remained constant until the last follow-up. Retrospective analysis for the presence of MICA antibodies revealed that the patient had DSA against MICA *027 at pre-transplantation with MFI of 3151. Post-transplant monitoring revealed an increase in MFI values over a period of time and was recorded to be 6225 at POD 180 [Figure 3]. However, no anti-HLA antibodies were detected and the patient remained asymptomatic with a well-functioning graft.
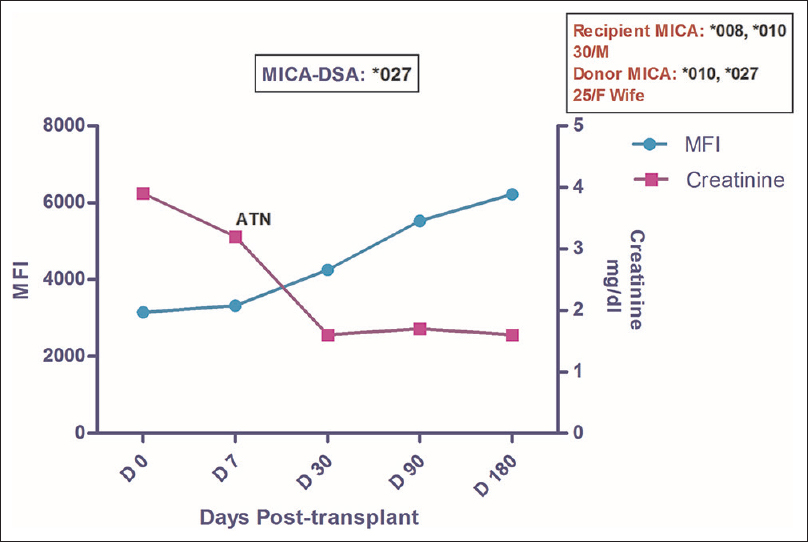
- Longitudinal DSA analysis of Case 3: The patient had DSA against MICA*027 at pre transplantation with MFI of 3151. He developed ATN on day 7 with a slight ↑ in MFI to 3325. Creatinine on day 7 was 3.2 which settled down to a baseline value of 1.6. No rejection episode was seen till the post-transplant follow-up of 18 months. No HLA-DSA was detected in this patient
Patients developing de novo DSA
Case 4
A 24-year-old male was transplanted against a negative CDC crossmatch and pre-transplant PRA of 7% with mother as the HLA haploidentical donor. No induction was given to the patient and he was maintained on TDI. His post-transplant creatinine settled to 1.6 mg/dl on POD 7. Post-transplant period was uneventful till POD 573 when he presented with an asymptomatic rise in creatinine to 4.8 mg/dl. Graft biopsy at this point of time revealed C4d + AMR. An analysis of the serum sample at the time of biopsy revealed the presence of DSA against MICA *008 (MFI = 8267). The patient was treated with plasmapheresis, IVIG and Bortezomib to which he responded well. His creatinine returned to a baseline value of 1.8 mg/dl while antibodies disappeared altogether from the circulation. On retrospective analysis, DSA against MICA *008 (MFI = 5406) was first detected in circulation at POD 180 [Figure 4]. No HLA antibody was ever detected in this patient by SAB assay.

- Longitudinal DSA analysis of Case 4: Patient had no HLA or MICA antibodies at pre transplant stage. DSA to MICA*008 was first detected on POD 180 with MFI of 5406. He developed AMR (C4d+) on POD 573. At this point of time the MFI value was 8267. He was treated successfully and no further episode of rejection seen till the post-transplant follow-up of 35 months
Case 5
A 32-year-old male was transplanted against a negative CDC crossmatch and PRA of 3.7% with mother as the HLA-haploidentical donor. No induction was given and the patient was maintained on TDI. Post-transplant creatinine settled down to 1.3 mg/dl by POD 30. Posttransplant period was uneventful till POD 357 when the patient developed asymptomatic rise in creatinine to 2.4 mg/dl. He was found to have C4d + AMR on graft biopsy done at this point of time. Antibody analysis at the time of biopsy showed HLA-DSA against HLA-B *35 with MFI value of 5606 and DSA against MICA *002 with MFI value of 5451. The patient was treated with plasmapheresis, IVIG and Bortezomib but did not respond and the graft was eventually lost. On analysis on stored sera, DSA against both HLA-B*35 (MFI of 2475) and MICA*002 (MFI = 1403) was first noticed on POD 90. Subsequent analysis showed a rise in MFI values against both alleles to 3816 and 3465 respectively on POD 180 [Figure 5].
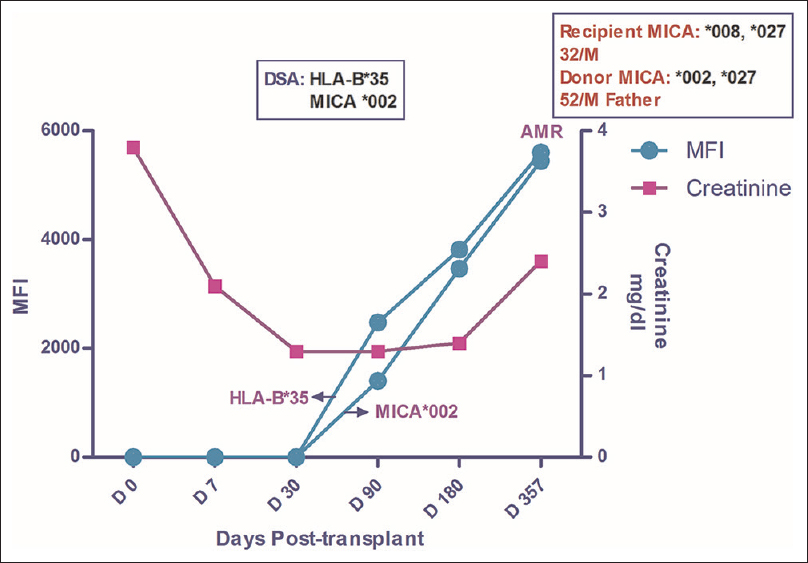
- Longitudinal DSA analysis of Case 5: Patient had no HLA or MICA antibodies at pre transplant stage. DSA to HLA-B*35 and MICA*002 was first detected on D 90 with MFI of 2475 and 1403 respectively. The MFI values kept on increasing till POD 357 when he developed AMR (C4d+). At the time of diagnosis of AMR, the MFI values for HLA and MICA DSA were 5406 and 2381 respectively. Ultimately the graft was lost in this patient. Creatinine at the time of first detection of DSA was 1.3 which rose to 2.4 when the biopsy was done
Discussion
Ever since Zwirner et al.[1] first demonstrated the expression of MICA antigens on endothelial cells, this molecule has attracted the much-needed attention of researchers as a possible target of graft dysfunction following solid organ transplantation. Of all the non-HLA antigenic targets, MICA has been the most polymorphic and extensively studied especially in renal allograft outcomes. Like for HLA, a prior graft could sensitize the patient for the MICA molecules leading to the development of anti-MICA antibodies. It has been suggested that pregnancy can also induce MICA antibodies while the role of pretransplant blood transfusions is not fully clear.[21011]
This is the first case series describing antibody-mediated rejection attributable to the presence of MICA DSA in live related donor, non-sensitized primary renal transplant recipients. Case 1 and 2 in our series demonstrate an association of pretransplant MICA-DSA (high MFI, range 10-15,000) with acute AMR in an accelerated manner. These results corroborate those of others,[79] although their patients were undergoing the second transplant and it is conceivable that the previous transplants could be acting as the main sensitizing event. Our patients, on the other hand are non-sensitized primary transplant recipients. Moreover, case no 1 actually presented with C4d negative AMR. This may suggest the possibility of both complement and non-complement mediated injury by MICA antibodies. Although the exact event leading to MICA sensitization in the above two cases is not clear, possibility of cross-reactivity with unidentified microorganisms cannot be ruled out.
Previous reports have suggested that both donor specificity as well as the antibody strength measured in terms of MFI is equally important for impact of graft function.[3679] This is evident in both case 1 and case 2 where the pretransplant MICA DSA had rather high MFI values of 13907 and 12945 respectively and both developed AMR as early as POD 5 and 12. In case 3, the patient developed MICA-DSA but the highest MFI recorded was 6225 at POD 180. The patient remained rejection free till 1 year of follow-up. This highlights the importance of recording MFI whereby high levels are generally associated with graft rejection.
On the other hand, the observed AMR in cases 4 and 5 is a result of de novo development of MICA antibodies. To that extent, this is the first case report suggesting a detrimental effect of de novo development of MICA-DSA. The presence of de novo DSA was defined as detection of antibodies at any time following transplantation on the sequential evaluation of sera in the recipients who were not found to have antibodies any time prior to transplant by single antigen bead (SAB) assay based on Luminex platform. It may be mentioned that the simultaneous presence of both anti-HLA and anti-MICA antibodies has earlier been shown to be highly detrimental.[1213] The case 4 in this series had both HLA as well as MICA DSA and developed AMR within one year of the transplant despite having peak MFI of as low as 2381 for MICA-DSA. This suggests that HLA and MICA antibodies act synergistically.
This case series highlights four important points in consideration of MICA DSA: i) currently employed pretransplant crossmatch procedures do not detect MICA DSA ii) higher MFI value of MICA DSA (>8000) is associated with rejection episodes iii) AMR due to MICA-DSA is amenable to conventional treatment of AMR and (iv) the series emphasizes the need for screening of MICA antibodies, on a case to case basis especially those directed against the donor.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Differential surface expression of MICA by endothelial cells, fibroblasts, keratinocytes, and monocytes. Hum Immunol. 1999;60:323-30.
- [Google Scholar]
- Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293-300.
- [Google Scholar]
- Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant. 2007;7:408-15.
- [Google Scholar]
- Major histocompatibility complex class I-related chain A allele mismatching, antibodies, and rejection in renal transplantation. Hum Immunol. 2011;72:827-34.
- [Google Scholar]
- Early renal graft function deterioration in recipients with preformed anti-MICA antibodies: Partial contribution of complement-dependent cytotoxicity. Nephrol Dial Transplant. 2016;31:150-60.
- [Google Scholar]
- Clinical relevance of major histocompatibility complex class I chain-related molecule A (MICA) antibodies in live donor renal transplantation – Indian experience. Scand J Immunol. 2020;92:e12923.
- [Google Scholar]
- Acute rejection associated with donor specific anti-MICA antibody in a highly sensitized pediatric renal transplant recipient. Pediatr Transplantation. 2011;15:E1-7.
- [Google Scholar]
- Clinical relevance of antibody development in renal transplantation. Ann N Y Acad Sci. 2013;1283:30-42.
- [Google Scholar]
- Acute antibody-mediated rejection in presence of MICA-DSA and successful renal re-transplant with negative-MICA virtual crossmatch. PLoS One. 2015;10:e0127861.
- [Google Scholar]
- Identification of MICA as a new polymorphic alloantigen recognized by antibodies in sera of organ transplant recipients. Hum Immunol. 2000;61:917-24.
- [Google Scholar]
- Major histocompatibility complex class 1 chain-related antigen A antibodies: Sensitizing events and impact on renal graft outcomes. Transplantation. 2010;90:168-74.
- [Google Scholar]
- Post transplant development of MICA and anti-HLA antibodies is associated with acute rejection episodes and renal allograft loss. Hum Immunol. 2007;68:362-7.
- [Google Scholar]
- HLA and MICA antibodies: Further evidence of their impact on graft loss two years after their detection. Clin Transpl. 2006;20:207-18.
- [Google Scholar]







