Translate this page into:
Mineral bone disease in maintenance hemodialysis patients: Association with morbidity and mortality
Address for correspondence: Dr. Georgi Abraham, 9/2, 15th Avenue, Harrington Road, Chetpet, Chennai - 600 031, Tamil Nadu, India. E-mail: abraham_georgi@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
There is a paucity of data on mineral bone disease in maintenance hemodialysis (MHD) patients from India. This retrospective analysis was undertaken on 858 (males: 599; females: 259) patients from two medical centers on MHD from 1998 to 2010. Age, gender, months on dialysis, hours per session of dialysis, hemoglobin, serum calcium, inorganic phosphorus, intact parathyroid hormone (iPTH), urine output, erythropoietin dosage per week, blood sugar, blood pressure, urea reduction rate, gain in fluid and fluid removed per session, serum albumin, alkaline phosphatase, vitamin D level, supplemental vitamin D and use of phosphate binder for therapy were documented. Overall, 191 patients died (22%) during the observation period. There was an 86% patient survival rate at 1 year on dialysis and an overall predicted 3-year survival rate of 78%. A relatively higher iPTH (P = 0.012), a need for vitamin D supplementation (P = 0.003), less hours on dialysis per session (P = 0.046) and a non-vegetarian diet (P = 0.022) were significantly associated with mortality.
Keywords
Asia
mineral bone disease
hemodialysis
Introduction
Chronic kidney disease (CKD) is a world-wide public health problem, with increasing prevalence.[1] It is associated with a number of abnormalities in mineral metabolism viz. hypocalcemia, hyperphosphatemia and abnormalities in vitamin D metabolism that result in functional calcitriol deficiency.[234] The major consequences of disordered mineral metabolism in CKD are secondary hyperparathyroidism (SHPT), metabolic bone disease including renal osteodystrophy and vascular calcification.[456]
The term CKD-mineral and bone disorder (CKD-MBD) can be used to describe a broad clinical syndrome that develops as a systemic disorder of mineral and bone metabolism due to CKD.[78] As such, circulating parathyroid hormone (PTH) levels have been used as a surrogate indicator of bone turnover, which are used together with measurements of serum calcium, phosphorus and alkaline phosphatase (ALP) levels to evaluate, diagnose and guide the treatment of CKD-MBD. However, the specificity of PTH as an indicator of bone turnover has been questioned.[910] Several other circulating biochemical markers of bone formation and resorption have been investigated as clinical indicators of bone turnover,[1112] but their clinical applicability remains to be established.
There is a lack of published data on CKD-MBD in Indian patients and on how the proposed interventions actually alter morbidity and mortality. Hence, this retrospective cohort study was undertaken to examine the relationship between calcium, phosphorus, intact PTH (iPTH), vitamin D levels and mortality in CKD patients undergoing hemodialysis.
Materials and Methods
The study included 858 patients from two medical centers on maintenance hemodialysis (MHD) (from January 1998 to January 2010 whose data were available for analysis). The inclusion criteria included all patients who started dialysis in that period including both incident and prevalent patients. Patients with acute kidney injury who required temporary dialysis and recovered renal function were excluded from the analysis.
Age, sex, vintage of dialysis, hours per session of dialysis, hemoglobin (Hb), serum calcium, serum inorganic phosphorus, serum iPTH, urine output, coronary artery disease, erythropoietin dosage per week, random blood sugar, blood pressure, urea reduction rate, gain in fluid and fluid removed per session, serum albumin, serum ALP, serum vitamin D level, supplemental vitamin D usage and use of phosphate binder for therapy were recorded. iPTH, 25-OH vitamin D3, calcium, phosphorus and albumin levels were measured on fasting samples prior to starting hemodialysis midweek. iPTH levels were measured in all patients using chemiluminescence assay. 25-OH vitamin D3 levels were measured in all patients by using radioimmunoassay method. The serum inorganic phosphorus and calcium values taken were the means of three estimates from blood drawn before a hemodialysis session after the patients were stable on dialysis for 3 months. The values of serum calcium were corrected to the serum albumin level before the mean was estimated ({observed calcium (mg/dl) + [albumin (40 g/L) − observed albumin]} ×0.02). Hb was estimated by drawing a pre-dialysis sample.
Patients were prescribed phosphorus binders if the serum phosphorus was above 4.5 mg/dl. The commonly used phosphorus binder were calcium acetate 667 mg tid, followed by calcium carbonate 500 mg, tid and lately, few were supplemented with sevelamer hydrochloride or sevelamer carbonate, 400 mg, tid, as an add-on therapy to calcium acetate and calcium carbonate.
The residual urine was measured 3 months after the initiation of dialysis. Diabetic control was achieved with either short and intermediate acting insulin, pioglitazone or glipizide and their HbA1c was measured at 3 months of initiation of dialysis and as often as it was necessary. Patients were given vitamin D if the 25-OH vitamin D3 levels were <30 ng/mL or if they had SHPT as suggested by the Kidney Disease Outcomes Quality Initiative (KDOQI) and the Kidney Disease Improving Global Outcomes (KDIGO) guidelines. Patients were supplemented with either alfacalcidol 0.25 μg/day, or calcitriol 0.25 μg/day, cholecalciferol 60,000 IU once a week by mouth. Inherently, the studies in India have shown that patients with different stages of CKD have low levels of 25-OH vitamin D3 levels compared with Caucasians or Blacks and hence the usual recommended dosage is 60,000 IU of cholecalciferol once a week along with alfacalcidol or calcitriol in patients with levels of <15 ng/mL. Repeat vitamin D estimates could not be done because of logistic reasons. Patients received epoetin alpha, epoetin beta or methoxy polyethylene glycol-epoetin beta (Mircera) along with intravenous iron sucrose. The dosage of epoeitin alpha and beta was at a mean of 4000 units weekly and Mircera 50 μg once in 2-4 weeks subcutaneously.
Statistical analysis was performed using SPSS [owned by IBM]. Differences in mean between continuous variables were done with one-way analysis of variance and post-hoc Tukey test. Continuous variables were analyzed by Chi-square test.
Results
Among the 858 patients (males: 599, females: 259) included from the two centers, Madras Medical Mission Hospital and TANKER Foundation, an all-cause mortality of 22% (191 patients) with a 1-year patient survival rate of 86% and a 3 year survival rate of 78% was reported [Figure 1]. All baseline demographic details are presented in Table 1. Most patients died due to cardiovascular complications. Relatively higher iPTH (P = 0.012) [Table 2], need for vitamin D supplementation (P = 0.003), less dialysis dosage (P = 0.046) and a non-vegetarian diet (P = 0.022) were significantly associated with mortality. Among patients who died, 76.5% had a serum phosphorus level more than 4.5 mg/dl (4.5-12 mg/dl) compared with those with a value <4.5 mg/dl (P = 0.50) but this trend was not seen with calcium levels <9.5 mg/dl (P = 0.634). The last known residual urine volume was 455 ± 453 mL/day and this was not significantly associated with mortality. The total number of patients who received an allograft was 291 (34%) and the average values of their biochemical parameters 3 months prior to transplant were taken for the purpose of this study.
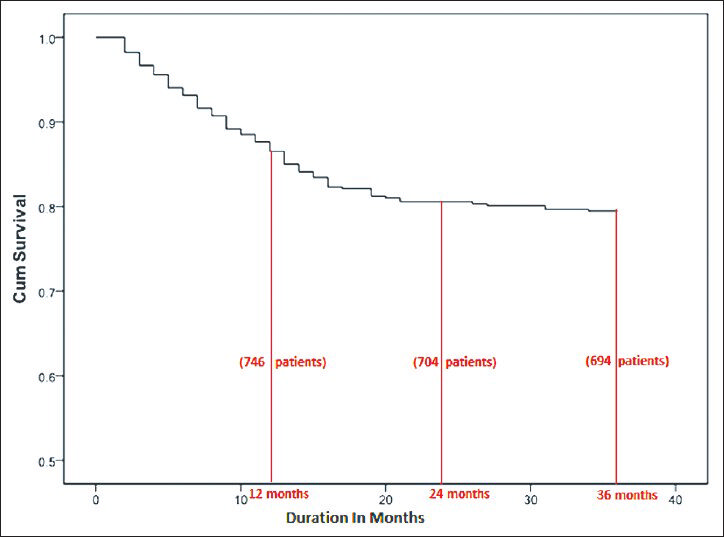
- Patient survival on hemodialysis


The cohort consisted of 364 diabetics (42.5%), however, diabetic status did not show a statistically significant association with mortality (P = 0.701).
Discussion
The life expectancy in India on an average is 69.89 years. For males, it is 67.46 years and 72.61 years for females. For dialysis patients, the life expectancy is significantly shortened. In a previous study, the mortality rate was 38.1% in the low socio-economic group and 4.2% in the high socio-economic status.[13] Although MHD prevents death from uremia, other comorbid conditions such as cardiovascular disease, diabetes, under-dialysis, infections, sepsis and MBD compound the issue of quality of life and survival. Some evidence suggests that mortality rates among dialysis patients have decreased over the last few years, suggesting that improvements in therapy may provide beneficial results[1413] There is no large registry data of hemodialysis patients in India.
The overall mortality in our study population was 22%. Factors associated with mortality in our study group included a relatively higher iPTH (P = 0.012), the need for vitamin D supplementation (P = 0.003), less hours on dialysis and thus a lower Kt/V (P = 0.046) and a non-vegetarian diet (P = 0.022).
Previous reports have suggested that serum iPTH or bone specific ALP can be used to evaluate bone disease as a metric for bone turnover rates. In our cohort of patients, 103 patients (12%) had a low bone turnover rate as shown by a serum iPTH of <100 pg/mL and 11 (1.3%) patients had a high bone turnover rate with a serum iPTH greater than 800 pg/mL. None of our patients required parathyroidectomy for refractory hyperparathyroidism. This highlights the fact that severe SHPT is a rare occurrence in our cohort of patients, which may be related to dietary habits and genetic factors currently unclear to the South Indian population.
The majority of our patients (86.7%) had an iPTH level recommended for patients with CKD on dialysis as suggested by the KDOQI and KDIGO.[12] The ALP levels in our cohort of patients did not show great elevation which suggests short duration of chronic kidney disease with not so profound MBD. Unfortunately, we could not measure bone specific ALP which is a more specific marker for bone turnover state. Bone biopsy was advocated by KDIGO in 2009 to diagnose bone disease, however, this is difficult to follow for many practicing nephrologists without access to specialized diagnostic and histopathological support services and bone biopsies are rarely performed in many parts of the world. There are now randomized control trials of therapeutic strategies in CKD bone disease derived from bone biopsy information.[15]
A relatively higher iPTH level has been associated with increased mortality in numerous studies as was also seen in our study population. The high iPTH may be the result of inadequate use of vitamin D, phosphorus binders and late jump start of dialysis in CKD patients in India. iPTH has been directly shown to have an independent effect on mortality in dialysis patients and this stands true for our population as well.[11] This likely reflects worsening renal function leading to progressive SHPT and increased mortality.
Most treatment guidelines suggest initiation of calcitriol or other vitamin D analogues with moderate rise in iPTH levels. Generic and relatively cheaper calcimimetics are available in India and hence combining vitamin D with calcimimetics may be an efficient modality, but data is lacking.
Substitution of vegetable protein for animal protein in normal subjects has a beneficial effect on renal hemodynamics. The vegetarian diet was associated with lower protein and phosphate intake in 20% of our dialysis patients. Furthermore, the vegetarian diet with exception of hard cheese, egg yolks, meat, poultry and fish is less acid producing and may have a protective effect on the bone and degree of hyperphosphatemia.[16] Thus, there is suggestive evidence that vegetarian diets may be advantageous in CKD-as traditional Indian non-vegetarian diet consists of meat, fish or egg 2-3 times a week-provided, essential nutritional elements are supplied through the diet. Even Indian non-vegetarians consume much lower protein compared with their counterparts in developed countries. Our study shows that a non-vegetarian diet is associated with a higher incidence of mortality: 25.6% mortality in vegetarians versus 39.4% mortality in non-vegetarians. Further, as the Indian non-vegetarian diet has little salt restriction, cardiovascular death is the cause for the excess mortality in our cohort.[1718] However, we are not able to fully explain the exact relationship between a non-vegetarian diet and mortality in our retrospective cohort of patients.
The European Renal Best Practice (ERBP) study working group acknowledges that the desirable ranges for plasma 25-OH vitamin D levels in CKD are not known, nor is the effect of physiological supplementation clearly established on important end points such as bone quality (density and microarchitecture), fracture rates, cardiovascular calcifications and cardiovascular and overall mortality tests.[15] Hence, it may be speculative to look at cardiovascular death correlating with vitamin D levels as there is no clear-cut evidence to suggest this.
In our cohort, only 28% of patients had vitamin D levels at or above 30 ng/mL and 13% had severe vitamin D deficiency of less than 6 ng/mL.
In a study by Matias et al. Post-repletion vitamin D concentrations if normal (>30 ng/mL) did not appear to be associated with short term harm which was expressed as biochemical end points only.[19] However, long term studies on mortality are lacking. Evidence for patient survival benefit for treating insufficient levels of 25-OH vitamin D in CKD have not yet definitely been demonstrated. But, in view of the low costs and therapeutic potential, they have been widely used in Indian patients with CKD well ahead of reaching stage 5 CKD. Our data seems to suggest that higher post-repletion vitamin D levels obtained seem to be associated with mortality in our population. This finding needs confirmation in larger randomized controlled clinical trials and if confirmed may lead to more prudent vitamin D supplementation and may require more accurate defining of the “normal” 25-OH vitamin D in the Indian CKD population.[19]
Our study showed a trend for higher mortality in those with serum phosphorus >4.5 mg/dl. However, the mortality was significantly related to high iPTH (P = 0.012), less hours on dialysis and thus a lower Kt/V (P = 0.046) and a non-vegetarian diet (P = 0.022). In developing economies like India where the diet which the dialysis patients consume are substantially low in protein and therefore, phosphorus. This has a direct impact on their serum phosphorus levels and concomitantly, the calcium phosphorus product compared to the diet in Africa, Europe, Far East and America. This may be due to inadequate dialysis as up to 80% of patients on dialysis have only <2 sessions of dialysis per week due to economic constraints and non-availability of state support [Figure 2]. This is a reflection of the cost containment by the patients themselves as nearly 65-80% of the patients have to pay out of pocket for dialysis, erythropoietin and other medications leading to increased morbidity and mortality, as only 1% of the gross domestic product is spent by the government for health care in India.

- Hours on dialysis per week
Our study showed that shorter time on dialysis and thus, probably, a lower Kt/Vurea was associated with increased mortality. A recent randomized trial showed that as compared with conventional hemodialysis, frequent hemodialysis was associated with favorable changes in the composite co-primary outcomes of death or 12-month change in left ventricular mass and death.[11] The association of shorter dialysis time per week with mortality in our patient population provides further evidence for a rethink on current dialysis practices which typically includes dialysis 2 or less commonly, 3 times a week. A longer duration of dialysis as shown by Chertow et al. may be beneficial in decreasing mortality and further studies are needed in this area before such a paradigm shift in practice can be contemplated. Hence, national policies have to be formulated regarding accessibility, quality and cost factor in MHD patients in India.
The cohort consisted of 364 diabetics (42.5%), however, diabetic status did not show a statistically significant association with mortality (P = 0.701). While diabetes is the most common cause of end-stage renal disease (ESRD) worldwide, data is scarce on how to best treat diabetic patients in ESRD. Blood glucose levels need to be well-controlled in these patients, several observational and one non-randomized interventional study showed that higher levels of HbA1c were associated with higher death rates. However, ESRD and dialysis significantly alters HbA1c levels, glycemic control and the excretion of anti-diabetic medication, making it difficult to monitor and treat diabetes effectively without increasing the risk of hypoglycemia.[20] We are unable to fully explain the lack of association with mortality in our patients and this needs further follow-up with a larger cohort.
The limitations of our study include the following: (1) Our study is limited by lack of estimation of markers of inflammation such as asymmetric dimethylarginine, high-sensitivity C-reactive protein, ferritin, fibronectin, haptoglobin, serum amyloid A which suggest higher oxidative stress and mortality.[21] (2) This is a cross-sectional retrospective cohort study and hence we did not analyze long term changes in serum values over time in association with morbidity and related factors. (3) Cardiac calcification scoring, bone biopsy, valvular and aortic calcification, carotid intimo-medial thickness were not done to correlate factors including fibroblast growth factor-23 to find high risk patients. (4) There is no clear-cut data on the cardiac status as serial echocardiogram details are not available in our patients. (5) The current recommendation by KDIGO and ERBP of screening incident dialysis patients using plain lateral X-ray of the abdomen for aortic calcification and valvular calcification by echocardiography was not undertaken, although there is fierce debate within nephrology about this approach and hence, we cannot comment on the vascular calcification in our cohort of dialysis patients.[2223]
Conclusion
Higher absolute values of serum phosphorus levels showed a trend for higher mortality, though not statistically significant. Higher iPTH levels, less hours on dialysis and a non-vegetarian diet were significantly associated with higher mortality.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Complications of chronic kidney disease: Anemia, mineral metabolism, and cardiovascular disease. Med Clin North Am. 2005;89:549-61.
- [Google Scholar]
- Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis. 2004;43:558-65.
- [Google Scholar]
- Prevalence and progression of peripheral arterial calcifications in patients with ESRD. Am J Kidney Dis. 2003;41:140-8.
- [Google Scholar]
- Why do dialysis patients develop a heart of stone and bone of china? Blood Purif. 2005;23:203-10.
- [Google Scholar]
- Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695-701.
- [Google Scholar]
- Clinical and laboratory features of aluminum-related bone disease: Differences between sporadic and “epidemic” forms of the syndrome. Am J Kidney Dis. 1985;6:342-7.
- [Google Scholar]
- Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis. 1998;31:607-17.
- [Google Scholar]
- Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1, 3, and 4 study. J Am Soc Nephrol. 2005;16:1788-93.
- [Google Scholar]
- Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520-8.
- [Google Scholar]
- Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208-18.
- [Google Scholar]
- Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245-52.
- [Google Scholar]
- K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis (42 Suppl 3):S1-201.
- [Google Scholar]
- Resource settings have a major influence on the outcome of maintenance hemodialysis patients in South India. Hemodial Int. 2010;14:211-7.
- [Google Scholar]
- United States Renal Data System. Excerpts from USRDS 2009 Annual Data Report. U.S. Department of Health and Human Services. The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Am J Kidney Dis (55 Suppl 1):S1.
- [Google Scholar]
- Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) chronic kidney disease-mineral and bone disorder (CKD-MBD) guidelines: A European Renal Best Practice (ERBP) commentary statement. Nephrol Dial Transplant. 2010;25:3823-31.
- [Google Scholar]
- Dietary acid load: A novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis. 2013;20:141-9.
- [Google Scholar]
- Chronic peritoneal dialysis in South Asia-Challenges and future. Perit Dial Int. 2008;28:13-9.
- [Google Scholar]
- Malnutrition and nutritional therapy of chronic kidney disease in developing countries: The Asian perspective. Adv Ren Replace Ther. 2003;10:213-21.
- [Google Scholar]
- Cholecalciferol supplementation in hemodialysis patients: Effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol. 2010;5:905-11.
- [Google Scholar]
- Managing diabetes in hemodialysis patients: Observations and recommendations. Cleve Clin J Med. 2009;76:649-55.
- [Google Scholar]
- Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: A journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89:1512-25.
- [Google Scholar]
- The case against routine screening for vascular calcification in chronic kidney disease. Semin Dial. 2010;23:280-2.
- [Google Scholar]
- Awareness of vascular calcification alters mineral metabolism management. Semin Dial. 2010;23:267-70.
- [Google Scholar]







