Translate this page into:
Modalities of Diagnosis and Management of Peritoneal Dialysis-related Hydrothorax Including Videothoracoscopy-assisted Repair: A Single-center Experience
Address for correspondence: Prof. Narayan Prasad, Department of Nephrology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Raebareli Road, Lucknow - 226 014, Uttar Pradesh, India. E-mail: narayan.nephro@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Continuous ambulatory peritoneal dialysis (PD)-related hydrothorax (PDRH) is uncommon; however, it is associated with a high discontinuation rate and morbidity. We report clinical characteristics, pleural fluid chemistry patterns, diagnostic modality, management options, and outcomes in 12 patients who have confirmed pleuroperitoneal communication after the inception of the PD program at our institute. The incidence of PDRH in our study was 0.64%. The interval between initiation of PD and hydrothorax ranged from 7 weeks to 40 weeks (average 20.6 weeks). Ten (83.3%) had right-sided, one (8.3%) left-sided, and one (8.3%) bilateral hydrothorax. Most patients (83.3%) had dyspnea with chest symptoms, but two (16.6%) patients were asymptomatic. All patients had confirmed communication either by peritoneal scintigraphy or computed topography peritoneography. PD had to be stopped in two patients and patients were shifted back to hemodialysis. Pleurodesis, through thoracostomy with tetracycline or betadine, was used for four patients. Three patients underwent video-assisted thoracoscopy (VATS) with surgical repair of the diaphragmatic defect, and one underwent VATS assisted talc pleurodesis. All four patients who underwent VATS repair of the defect had successful outcomes. With availability and experience with VATS, most patients had successfully returned to PD with no recurrence and with minimal morbidity.
Keywords
Hydrothorax
peritoneal dialysis
videothoracoscopy
Introduction
Continuous ambulatory peritoneal dialysis (PD) is an effective and established renal replacement modality for patients with end-stage renal disease (ESRD). Out of various noninfectious complications, CAPD-related hydrothorax (PDRH) is an uncommon event. It occurs due to pleuroperitoneal communication. Though incidence is about 1.6–10% (1, 2), it is associated with a high discontinuation rate of PD and morbidity. Diagnosis is easy; however, it requires a high index of suspicion, particularly in a patient with progressive dyspnea with low ultrafiltration. The condition should be readily differentiated from other causes of pleural effusion, such as congestive cardiac failure, hypoalbuminemia, or fluid overload state, all being a common clinical association in ESRD patients on PD. Herewith, we report a case series of 12 patients with PDRH treated with various treatment modalities, including video-assisted thoracic repair.
We reviewed our hospital CAPD database of patients who have been started on PD from 1998 to 2018. Of the total of 1876 patients, a total of 12 cases (0.64%) had confirmed pleuroperitoneal communication. The clinical data, initial diagnosis, pleural fluid analysis and blood investigations, diagnostic modality, management, and outcomes were analyzed for all patients.
Cases details
Of the 12 cases Table 1 during the study period, 9 (75%) were males, and 3 (25%) were females, with a median age of 53.5 years (33-75 years). Among causes of ESRD, six (50%) had diabetic nephropathy, two (16.6%) chronic glomerulonephritis, two (16.6%) chronic interstitial nephritis, and one (8.3%) had acute cortical necrosis. The most common clinical presentations were gradually increasing shortness of breath in ten (83.3%), chest pain or discomfort in eight (66.6%), and inadequate ultrafiltration in four (33.3%) patients. In two (16.6%) cases, pleural effusion was detected incidentally on routine chest radiography performed for mild cough. One patient had associated consolidation of lungs and empyema thoracis. The interval between initiation of PD and hydrothorax ranged from 7 weeks to 50 weeks (average 20.6 weeks, in 6 patients; after 20 weeks). Ten (83.3%) had right-sided, one (8.3%) had left-sided, and one (8.3%) had bilateral hydrothorax. Most patients with pleuroperitoneal communication had new-onset pleural effusions by chest radiography after 4 months of the start of PD. In four patients, pleural effusion developed within 2 months, whereas three patients had effusion detected after 10 months of initiation.
| Patient | Gender | Age (yrs) | BMI (kg/m2) | Cause of ESRD | Clinical features at presentation | Duration of PD before the event | Side of hydrothorax |
|---|---|---|---|---|---|---|---|
| 1 | Male | 47 | 25 | DN | Dyspnea, chest pain | 7 weeks | Right |
| 2 | Male | 33 | 21 | ACN | Poor UF, dyspnea, chest heaviness | 50 weeks | Bilateral, initially on the right side, later on, left side after one week of right pleurodesis |
| 3 | Male | 58 | 26 | DN | Poor UF, dyspnea, chest pain | 42 weeks | Right |
| 4 | Male | 44 | 21 | DN | Asymptomatic, detected on routine chest x-ray | 20 weeks | Right |
| 5 | Male | 66 | 30 | ?CGN | Dyspnea, chest pain | 17 weeks | Right |
| 6 | Male | 75 | 28 | DN | Chest pain, fever, cough with expectoration | 13 weeks | Right |
| 7 | Male | 54 | 20 | ?CGN | Asymptomatic, detected on routine chest x ray | 10 weeks | Right |
| 8 | Female | 49 | 29 | ?CIN | Chest pain, dyspnea | 12 weeks | Left |
| 9 | Female | 53 | 23 | ?CIN | Dyspnea, dry cough | 7 weeks | Right |
| 10 | Male | 48 | 26 | DN | Dyspnea, cough with purulent expectoration, consolidation, empyema, sepsis | 20 weeks | Right |
| 11 | Female | 58 | 18 | ?CIN | Dyspnea, chest heaviness | 10 weeks | Right |
| 12 | Male | 57 | 26 | DN | Poor UF, chest pain, dyspnea | 40 weeks | Right |
Pleural fluid characteristics
The pleural fluid characteristics have been shown in Table 2. All patients underwent diagnostic pleurocentesis, and seven (58.3%) patients required therapeutic removal of 1.2 to 2.5 liters of fluid. In 11 (91.6%) cases, fluid was characterized as transudative according to one or more of Lights criteria. In one patient, effusion was categorized as exudative with culture positive for Staphylococcus aureus and empyema requiring multiple paracenteses with tube thoracostomy and prolonged course of intravenous antibiotics. Eight (66.6%) patients had pleural fluid to serum glucose gradient of more than 100 mg/dl, whereas only one had a value of less than 50 mg/dl, and four (33.3%) patients had a value between 50 and 100 mg/dl. Pleural fluid to serum glucose ratio was more than 1 in all (100%) cases. Total cell counts were within 5 to 100 per HPF except for one instance of empyema where counts were higher. Most of the pleural fluid but for one sample was lymphocyte-predominant and culture-negative for bacteria, fungus, and mycobacterium. In two patients initially, empiric antitubercular therapy was started by a local physician, which was stopped after confirmation of peritoneal dialysis-related effusion.
| Glucose pleural/serum | Pleural fluid to serum glucose gradient | Pleural fluid to serum glucose ratio | Protein pleural/serum | Pleural fluid to serum protein ratio | LDH pleural/serum | LDH pleural/serum ratio | TLC (Polymorph/Lymphocyte) |
|---|---|---|---|---|---|---|---|
| 304/109 | 195 | 2.78 | 1.0/4.6 | 0.21 | NA | NA | 5 (0/100) |
| 251/109435/88 | 142347 | 2.304.90 | 1.3/4.21.9/6.5 | 0.300.29 | NANA | NANA | 100 (20/80)15 (25/75) |
| 436/270 | 166 | 1.61 | 2.8/5.1 | 0.34 | 139/450 | 0.30 | 100 (20/80) |
| 420/200 | 220 | 2.10 | 3.1/6.8 | 0.45 | NA | NA | 10 (20/80) |
| 320/120 | 200 | 2.66 | 2.0/5.6 | 0.35 | 220/640 | 0.34 | 5 (0/100) |
| 190/89 | 101 | 2.13 | 2.3/6.8 | 0.33 | 137/328 | 0.41 | 70 (0/100) |
| 143/99 | 44 | 1.44 | 2.9/5.9 | 0.49 | 201/580 | 0.34 | 70 (3/97) |
| 162/84 | 78 | 1.92 | 2.0/6.4 | 0.31 | 128/304 | 0.42 | 20 (10/90) |
| 255/103 | 152 | 2.47 | 1.8/6.6 | 0.27 | 145/279 | 0.52 | 30 (5/95) |
| 276/204 | 74 | 1.35 | 3.4/5.3 | 0.64 | 420/575 | 0.73 | 340 (95/5) |
| 150/79 | 71 | 1.89 | 2.6/6.0 | 0.43 | 210/540 | 0.38 | 75 (30/70) |
| 170/90 | 80 | 1.88 | 1.1/4.2 | 0.26 | 168/326 | 0.51 | 15 (10/90) |
Imaging and treatment modality
The summary of diagnostic and treatment modalities is shown in Table 3. Nuclear scintigraphy was performed in nine (75%) cases, computed tomography (CT) peritoneography in five (41.6%) (representative [Figure 1]), and both nuclear and CT peritoneography in two (16.6%) to confirm the diagnosis of pleuroperitoneal communication. In order to repair the leak, four of them underwent VATS. Two of the patients with PDRH (16.6%) stopped PD, and Tenckhoff catheter was removed as they were not willing to continue PD and shifted back to hemodialysis. Seven (58.3%) patients underwent therapeutic thoracentesis, followed by pleurodesis in five (41.6%) patients with tetracycline in four and betadine in one patient. One patient had a recurrence of pleural effusion on the left side after successful pleurodesis of the right side within 7 days, for which repeat pleurodesis was done on the left side after 2 weeks. Four patients underwent VATS with suturing of the diaphragmatic defect (representative [Figure 2]), and one had VATS assisted talc pleurodesis as well. In all ten cases, willing to continue, PD was successfully restarted after 3-5 weeks of the break-in period after the procedure. All four (100%) patients who underwent VATS repair of the defect had successful outcomes, three of patients still on peritoneal dialysis without recurrence of effusion, and one patient continued for 28 months till she got a renal allograft transplant. None of our patients had undergone open thoracotomy.
| Patient | Confirmation of diagnosis | Management | Outcome |
|---|---|---|---|
| 1 | Tc-99m scintigraphy | PD stopped, shifted on HD | Effusion resolved, died of sepsis after 20 months |
| 2 | Tc-99m scintigraphy | Antitubercular drugs were stopped, PD withheld, thoracostomy, tetracycline pleurodesis on right side initially, then on the left side after two weeks later | Effusion developed on the left side after one week, PD restarted after one month of bilateral pleurodesis, no recurrence, continued on PD for 4 yrs, died of CAPD peritonitis |
| 3 | Tc-99m scintigraphy | Antitubercular drugs were stopped, PD withheld, thoracostomy, tetracycline pleurodesis | CAPD restarted after one month, no recurrence, continued of CAPD for 32 months, died of CAPD peritonitis |
| 4 | Tc-99m scintigraphy | PD withheld, tetracycline pleurodesis | CAPD restarted after 2 months, no recurrence, continued CAPD for 8 months, underwent renal transplantation |
| 5 | Tc-99m scintigraphy | PD withheld, tetracycline pleurodesis | Recurrence of effusion after 3 months, CAPD stopped, catheter removed, shifted on HD, lost to follow up |
| 6 | Tc-99m scintigraphy | PD stopped, shifted on HD | Effusion resolved, lost to follow up |
| 7 | Tc-99m scintigraphy | PD withheld, betadine pleurodesis, the second session of pleurodesis done after two weeks | CAPD restarted after three weeks, no recurrence, continued on CAPD for 34 months, died of CAPD peritonitis |
| 8 | CT peritoneography | PD stopped, shifted on HD, VATS repair of diaphragmatic defect with sutures | CAPD started after one month, no recurrence, continued on CAPD for 28 months, received a renal allograft |
| 9 | Tc-99m scintigraphy, CT peritoneography | PD stopped initially, shifted on HD for 5 months, recurrence of effusion on restart of CAPD within seven days, VATS repair | CAPD restarted after three weeks, no recurrence, continuing CAPD |
| 10 | CT peritoneography | CAPD stopped, thoracostomy for one month, antibiotics for empyema, shifted on HD for 3 months, recurrence of effusion on restart of CAPD, catheter removed | Effusion resolved, permanently shifted on HD, pleural thickening with lung fibrosis, died of sepsis after 3 months |
| 11 | CT peritoneography | CAPD stopped for 3 months, recurrence of effusion on restart of CAPD after two weeks, VATS repair | CAPD restarted after three weeks, no recurrence, continuing CAPD |
| 12 | CT peritoneography, Tc-99m scintigraphy | CAPD stopped initially, shifted on HD for one month, VATS repair | CAPD restarted after one month, no recurrence, continuing CAPD |
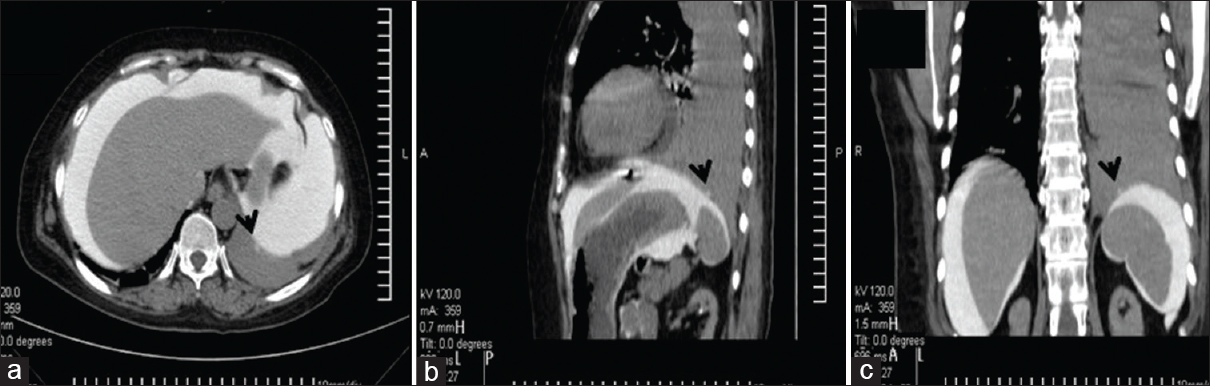
- (a) Cross-section image of CT peritoneography showing the leak point on the diaphragm (black arrow); (b) coronal section image showing defect on the dome of diaphragm; and (c) hydrothorax on the left side
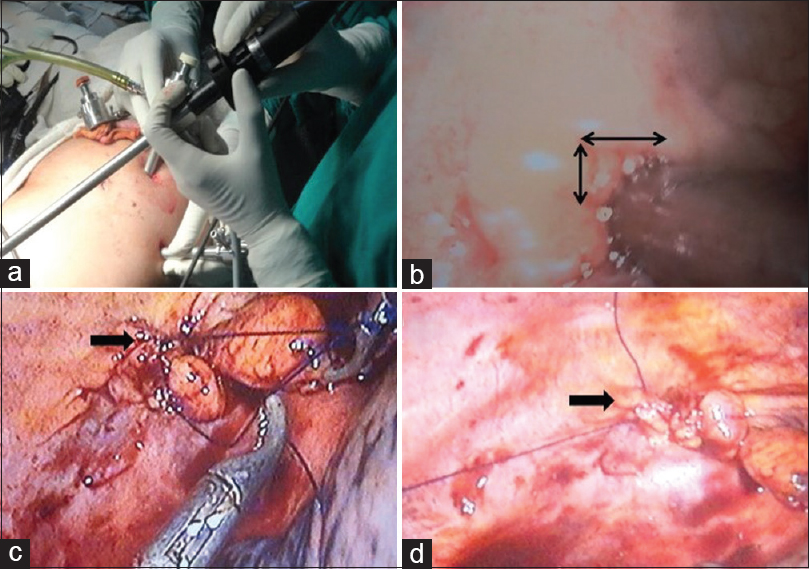
- (a) Showing the procedure of video-assisted thoracoscopic ports and method; (b) identifying opening at diaphragm; (c) process of taking the suture around the opening defect; and (d) finally closure suture of the defect
Discussion
Although PDRH is not life-threatening, however, it is associated with morbidity, hospitalization, and a high probability of discontinuation (50%). The incidence of PDRH varies between 1.6 and 10%[12] in adults, and about 3% in a pediatric series.[3] Our center observed an incidence of 0.64%, which is lower than reported in other studies. In our case series, of the 12 patients, only two patients were shifted to HD as they were not willing to continue PD and showed an unwillingness to be investigated and repair of the defect.
Like other studies, the most common clinical presentations were shortness of breath and chest discomfort with reduced UF. However, the clinical presentation may be asymptomatic in about 25% of cases and detected incidentally by routine chest radiography.[1] It manifests as right-sided hydrothorax in 88% within the first few months.[1] Left-sided and bilateral hydrothorax are uncommon. PDRH results due to the migration of dialysis fluid from the peritoneal cavity under positive pressure into the pleural space, which has negative pressure.[4] Increased intraabdominal pressure (IAP) arises either transiently or permanently due to PD solution, which increases further during coughing and straining. IAP reaches 120 – 150 cm H2O with coughing or straining versus 0.5–2.2 cm H2O in controls and 2–10 cm H2O in patients with PD fluid in the peritoneal cavity. Patients with ADPKD have a higher incidence of hydrothorax, possibly due to higher IAP.[5] The exact mechanism by which peritoneal dialysate crosses the diaphragm is not known, possibly due to a pleuroperitoneal communication (congenital or acquired) and/or by lymphatic transport along the aorta or thoracic duct.[67] Congenital defects (i.e., pneumatoenteric recess, infracardiac bursa) explain the preponderance of right-sided hydrothorax because left-sided defects are covered by the heart and pericardium, thereby reducing the possibility of a leak on the left side. In addition, abnormalities in subpleural lymphatic structures predominate in the right hemidiaphragm.[67]
Effusion is usually transudative in nature if not secondarily infected. The condition should be differentiated from other causes of transudative pleural effusion commonly present in the CKD population, such as congestive cardiac failure, hypoalbuminemia of nephrotic state, or cirrhosis of the liver and fluid overload state. Failure to recognize a pleuroperitoneal communication associated with hydrothorax may lead to the inadvertent prescription of hypertonic dialysis fluid exchanges, which may further increase intraperitoneal pressure and aggravate the hydrothorax. It may also unnecessarily lead to the prescription of diuretics or antibiotics, sometimes antitubercular drugs.
However, pleurocentesis, which is easy and inexpensive, is often done for diagnostic purposes or sometimes therapeutically for symptomatic relief. Biochemical and microbiological analysis of pleural fluid is essential to ascertain the transudative nature of effusion and to exclude exudative causes, such as bacterial infections, tuberculosis, and malignancy. Effusion has a low cell, and protein content and Light's criteria for transudative effusion usually holds true in 90% of cases.[89] Although glucose level in pleural fluid is similar to PD fluid and is much higher than serum value, there is no consensus on precise cut off values that can differentiate PDRH from other causes. Pleural fluid glucose may vary with the size of the defect, PD fluid glucose, and reabsorption of glucose by visceral pleura and lymphatics. The PF-S glucose gradient of 50–100 mg/dl is highly suggestive, and a gradient of >100 mg/dl is almost diagnostic PDRH. A PF-S glucose gradient of less than 50 mg/dl may be present in 20% of PDRH. Alternatively, pleural fluid to serum (PF/S) glucose ratio of >1 is more consistent and sensitive for PDRH, whereas the ratio is <1 in other causes of transudative effusion and is <0.5 in exudative effusions. To improve the accuracy of PF-S glucose gradient and PF/S glucose ratio, it is suggested that a pleural tap should be done using a 4.25% dextrose solution within 1 hour of exchange with the patient remaining supine after the exchange.[89]
Confirmation and localization of pleuroperitoneal communication can be done by various modalities. Discoloration of the dialysate with diluted dyes (methylene blue or indocyanine green) followed by thoracocentesis had been abandoned due to the high incidence of chemical peritonitis.[10] Similarly, the detection of icodextrin in the pleural fluid after using it as PD fluid is a simple method to confirms the diagnosis. Peritoneal scintigraphy using Tc-99m macro aggregated albumin or sulfur colloid and detecting movement of the isotope above the hemidiaphragm on the side of the defect was used in the center with nuclear medicine facility.[111213] CT peritoneography with intraperitoneal iodinated contrast has become the diagnostic modality of choice for PDRH. Besides, being sensitive, it can often localize the site of the leak. Magnetic resonance imaging with or without contrast has also been used to diagnose leaks.
Conservative management options include shifting the patient to hemodialysis either permanently or temporarily.[14] This alone may be sufficient in 53% of cases.[15] A large pleural effusion may require therapeutic thoracentesis for symptomatic relief.[1617] Early leaks with minor defects may heal spontaneously by withholding PD for 4–6 weeks.[18] Conventional pleurodesis or sclerotherapy is performed under local anesthesia through a thoracostomy tube using sclerosing agents.[19] Talc, tetracycline, betadine, fibrin glue, autologous blood all have been used as sclerosant with varying degrees of success. Once the pleural cavity is drained, sclerosant with 1% lignocaine diluted in 50–70 ml of normal saline is administered through a tube, and the patient is positioned lying left, supine, and then to the right for 15 min each then patient is kept flat for 24 hrs. Pain control is usually achieved by both local and systemic analgesics, including opioids. PD is restarted after 2–4 weeks after pleurodesis. The procedure is associated with about 48% success rate in a study.[1920]
Open thoracotomy with the repair of the diaphragmatic defect is the most definitive procedure with high success rates (100%) even for larger defects.[1415] Usually, this procedure is invasive and associated with high postoperative morbidity in ESRD patients; hence is no longer preferred and used only in exceptional cases.
The VATS-assisted repair has become the procedure of choice as it is both diagnostic and therapeutic and is associated with minimal morbidity.[212223] We have successfully performed VATS repair in the last four patients in our series. It permits direct visual identification and repair of diaphragmatic defects. Repair methods can include surgical stapling or suturing, use of a proline mesh or a Teflon patch, use of fibrin glue, or pleurodesis with a sclerosing agent. Intraoperative evaluation for air or fluid leakage assures closure of a pleuroperitoneal communication.[2425] VATS allows even distribution of sclerosant like talc (or tetracycline or fibrin glue) between pleura and hence better success rates (80-100%) and less pain compared to conventional pleurodesis. Of the four patients with VATS, one had VATS-assisted sclerotherapy in our series PD usually can be started after 2–4 weeks after the procedure.[2126]
Conclusion
Diagnosis of PDRH is easy but requires a high index of suspicion. The condition should be differentiated from other causes of pleural effusion. Management includes temporary withholding of PD for a few weeks, pleurodesis by tube thoracostomy, open thoracotomy, and VATS repair or assisted pleurodesis. With availability and experience with VATS, most patients have successfully returned to PD with no recurrence and with minimal morbidity hence should be considered the modality of choice if conservative management fails.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Acute hydrothorax in continuous ambulatory peritoneal dialysis—a collaborative study of 161 centers. Am J Nephrol. 1989;9:363-7.
- [Google Scholar]
- Patient characteristics associated with defects of the peritoneal cavity boundary. Perit Dial Int. 2005;25:367-73.
- [Google Scholar]
- Management of peritoneal dialysis-induced hydrothorax in children. Am Surg. 1996;62:820-4.
- [Google Scholar]
- Complications of peritoneal dialysis related to increased intra-abdominal pressure. Adv Perit Dial. 2003;19:130-5.
- [Google Scholar]
- Increased incidence of hydrothorax complicating peritoneal dialysis in patients with adult polycystic kidney disease. Nephrol Dial Transplant. 1994;9:832-3.
- [Google Scholar]
- Traumatic pleural leak in peritoneal dialysis. Nephrol Dial Transplant. 2001;16:1526.
- [Google Scholar]
- Hydrothorax complicating peritoneal dialysis: Diagnostic value of glucose concentration in pleural fluid aspirate. Perit Dial Int. 2002;22:525-8.
- [Google Scholar]
- Low pleural fluid-to-serum glucose gradient indicates pleuroperitoneal communication in peritoneal dialysis patients: Presentation of two cases and a review of the literature. Nephrol Dial Transplant. 2012;27:1212-9.
- [Google Scholar]
- Methylene blue as a cause of chemical peritonitis in a patient on peritoneal dialysis. Clin Nephrol. 1995;43:136-7.
- [Google Scholar]
- Peritoneal scintigraphy in the diagnosis of complications associated with continuous ambulatory peritoneal dialysis. Clin Nucl Med. 2003;28:70-1.
- [Google Scholar]
- Peritoneal scintigraphy for the assessment of dialysate leakage in patients on continuous ambulatory peritoneal dialysis. Ann NuclMed Sci. 2001;14:11-8.
- [Google Scholar]
- Massive hydrothorax complicating peritoneal dialysis. Isotopic investigation (peritoneopleural scintigraphy) Clin Nucl Med. 1993;18:498-501.
- [Google Scholar]
- The management of hydrothorax in continuous ambulatory peritoneal dialysis. Perit Dial Int. 1990;10:271-4.
- [Google Scholar]
- Management options for hydrothorax complicating peritoneal dialysis. Semin Dial. 2003;16:389-94.
- [Google Scholar]
- Hydrothorax in a patient receiving continuous ambulatory peritoneal dialysis: Successful treatment with intermittent peritoneal dialysis. Arch Intern Med. 1982;142:1571-2.
- [Google Scholar]
- Spontaneous resolution of hydrothorax in continuous ambulatory peritoneal dialysis. Nephron. 1992;61:247-8.
- [Google Scholar]
- Hydrothorax: Pleural effusion associated with Peritoneal dialysis. Perit Dial Int. 2010;30:13-8.
- [Google Scholar]
- Hydrothorax in peritoneal dialysis. Effective treatment with pleurodesis. Perit Dial Int. 1998;18:657-8.
- [Google Scholar]
- Hydrothorax in continuous ambulatory peritoneal dialysis: Successful treatment with intrapleural tetracycline and a review of literature. Am J Kidney Dis. 1985;5:136-40.
- [Google Scholar]
- Thoracoscopic pleurodesis for massive hydrothorax complicating CAPD. Perit Dial Int. 1991;16:421-5.
- [Google Scholar]
- Long-term follow-up of thoracoscopic pleurodesis for hydrothorax complicating peritoneal dialysis. Ann Thorac Surg. 2002;74:218-21.
- [Google Scholar]
- Video-assisted thoracoscopic talc pleurodesis is effective for maintenance of peritoneal dialysis in acute hydrothorax complicating peritoneal dialysis. Nephrol Dial Transplant. 2003;18:804-8.
- [Google Scholar]
- Videoassisted thoracoscopic treatment for pleuroperitoneal communication in peritoneal dialysis. Eur J Cardiothorac Surg. 2001;20:205-7.
- [Google Scholar]
- Acute hydrothorax in CAPD. Early thoracoscopic (VATS) intervention allows a return to peritoneal dialysis. Nephron. 2002;92:725-7.
- [Google Scholar]
- Video-assisted thoracoscopic surgery in continuous ambulatory peritoneal dialysis-related hydrothorax. Kidney Int. 2008;74:136.
- [Google Scholar]







