Translate this page into:
Monoclonal Gammopathy of Renal Significance (MGRS) – Case Series from a Tertiary Center in Kerala
Corresponding Author: Prof. Thomas Mathew, Senior Consultant and Head of Department, Department of Nephrology, Baby Memorial Hospital, Kozhikode, Kerala, India. E-mail: drmtmathew@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Padmanabhan P, Jayameena P, Mampilly N, Francis S, Sherif A, George S, et al. Monoclonal Gammopathy of Renal Significance (MGRS) – Case Series from a Tertiary Center in Kerala. Indian J Nephrol 2024;34:59-63. doi: 10.4103/ijn.ijn_329_22
Abstract
Monoclonal gammopathy of renal significance (MGRS) has gained importance because identifying the monoclonal deposit and addressing it, rather than treating renal dysfunction as the primary pathology, has salvaged the patients from progressing into end-stage renal disease. Since it affects elderly population, there could be a propensity to misdiagnose them with cardiorenal syndrome. We present four patients of MGRS diagnosed from our center. They presented with proteinuria or unexplained renal dysfunction. Three of the patients were diagnosed to have amyloidosis, of which two had lambda-type and one had kappa amyloidosis. The fourth patient had fibrillary glomerulonephritis with kappa restriction, further evaluation of which led to diagnosis of chronic lymphocytic leukemia. Absence of “M” band in protein electrophoresis and a normal bone marrow study should not stop physicians from further evaluation. Quantitative serum immunofixation electrophoresis and electron microscopic examination of renal biopsy have become a comprehensive diagnostic tool in such patients.
Keywords
Fibrillary glomerulonephritis
kappa amyloidosis
monoclonal gammopathy of renal significance (MGRS)
serum immunofixation electrophoresis
Introduction
The presence of a monoclonal immunoglobulin or its component, produced by a B cell or plasma cell clone is termed as monoclonal gammopathy. As long as it does not satisfy the criteria defining the “tumor burden” or affect organ function, it is termed monoclonal gammopathy of unknown significance (MGUS). A monoclonal protein may be toxic due to its ability to bind other proteins, misfold, or deposit in tissues. When monoclonal gammopathy–related kidney damage occurs in the absence of overt malignancy, the hematologic disorder is categorized as monoclonal gammopathy of renal significance (MGRS).1
MGRS-associated kidney diseases are characterized by three features: (1) these diseases do not respond well to immunosuppressive regimens used in the treatment of autoimmune nephropathies; (2) affected patients have a very high rate of recurrence after kidney transplantation (approximately 90%) if the monoclonal gammopathy is not eliminated before or immediately after transplantation; and (3) affected patients are at risk for progression to the corresponding hematologic cancer.2
The treatment of such dysproteinemic kidney diseases is directed at the underlying clonal proliferative disorder, with the goal of achieving a hematologic response, that is, improvement or normalization of paraprotein levels in blood and urine, and thus attaining overall survival and organ-specific response.1
Case Series
Case 1
A 46-year-old male presented with nephrotic syndrome. He had proteinuria of 2.8 g over 24 h, no hematuria, and leukocytosis (17,700/mm3) with 75% lymphocytosis. Urea was 57.7 mg/dl, serum creatinine 3.1 mg/dl, and uric acid was 8.2 mg/dl. Peripheral smear and vasculitic profile were normal. Renal biopsy revealed fibrillary glomerulonephritis (GN) with kappa restriction [Table 1]. DNAJB9 could not be done due to lack of resources.
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age (years) | 46 | 70 | 66 | 51 |
| Pretreatment urine protein creatinine ratio |
2.82 | 1.51 | 5.55 | 11.4 |
| Creatinine | 3.1 | 1.8 | 1.1 | 0.9 |
| Albumin: globulin ratio | 0.8 | 1.2 | 0.4 | |
| ECHO | Not done | Type 3 diastolic dysfunction with concentric LVH | Hypertrophic nonobstructive cardiomyopathy | Not done |
| Free kappa, mg/l (3.3-19.4) | 146.94 | 106 | 12.82 | 22.7 |
| Free lambda, mg/l (5.71-26.3) | 36.09 | 11.8 | 114.24 | 108.92 |
| Kappa/lambda (K/L) ratio (0.26-1.65) | 4.07 | 8.9 | 0.11 | 0.21 |
| Monoclonal (M) protein | Absent | Absent | Positive (0.51 g %) in beta region |
Absent |
| Renal biopsy (LM) | Focal mesangial proliferation. [Figure 1] |
Congo-positive nodular mesangial deposits with apple green birefringence. [Figure 3] | Mild mesangial hypercellularity |
Mesangial hypercellularity with mesangial expansion by a fibrillary material |
| Renal biopsy IF | C3-2+, C1q-1+, IgG-1+ Kappa restriction |
IgG-2+ Kappa restriction | Lambda restriction | C3-3+lambda restriction |
| Renal biopsy EM | Nonbranching, randomly oriented fibrils 10.1-19.6 nm in diameter. [Figure 2] | Not done | Focal streaming arrangement of randomly oriented fibrils 9-12 nm in diameter. [Figure 4] | Focal streaming arrangement of randomly oriented fibrils 9-12 nm in diameter. [Figure 5] |
| Renal biopsy diagnosis | Fibrillary GN | AL amyloidosis | AL amyloidosis | AL amyloidosis |
| Bone marrow | Chronic lymphocytic leukemia |
Normal | Normal | Normal |
| Treatment | Rituximab, steroids | CyBorD induction with bortezomib maintenance | CyBorD, intolerant to bortezomib | Daratumab, cyclophosphamide, bortezomib and dexona |
| Post treatment proteinuria | 1442 | 1400 | ||
| Creatinine | 1 | 2.5 | 1 | |
| Free kappa, mg/l (3.3-19.4) | 44.214 | 53 | 9.3 | 14.29 |
| Free lambda, mg/l (5.71-26.3) | 27.214 | 51 | 7.6 | 15.15 |
| K/L ratio (0.26-1.65) | 1.62 | 1 | 1.22 | 0.94 |
CyBorD=cyclophosphamide, bortezomib, and dexona, GN=glomerulonephritis, LM=Light microscopy, IF=Immunofluorescence, EM=Electron microscopy
Serum quantitative immunofixation electrophoresis (SIFE) showed elevated serum kappa and lambda levels with a higher kappa/lambda ratio; M band was absent [Table 1]. Repeat peripheral smear examination showed smudge cells. A bone marrow study was consistent with chronic lymphocytic leukemia (CLL). Immunophenotyping showed 70% abnormal population of B cells negative for both kappa and lambda and for FMC7 and expressing dim CD20, CD19, dim CD22, CD200, CD5, CD23. The fluorescence in situ hybridization (FISH) test was positive for 13q14.2 and negative for deletion 6q, deletion 11 q, trisomy 12, Immunoglobulin heavy chain locus (IGH) rearrangement, and deletion 17p [Figures 1 to 5].
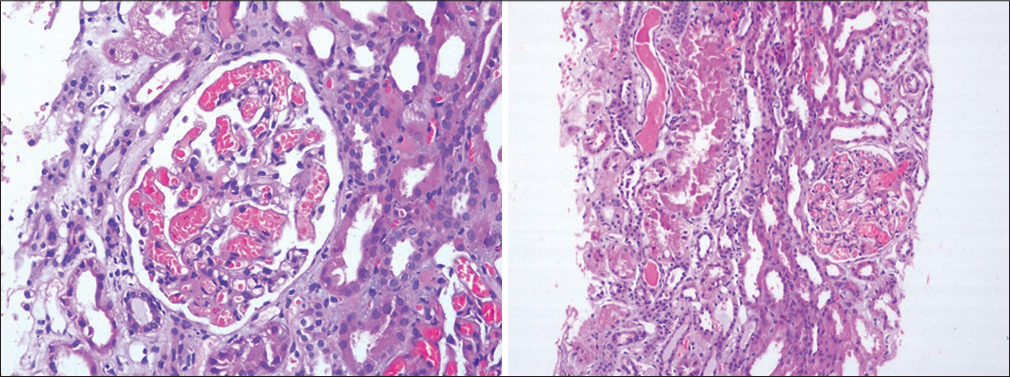
- H&E-stained image showing focal mesangial proliferation and capillary lumen filled with red blood cells. H&E = hematoxylin and eosin 20X.

- Electron microscopy showing significant foot process effacement. Several mesangial and paramesangial areas showing aggregates of nonbranching, randomly orientedfibrils. No electron dense deposits identified.

- Glomerulus and blood vessel showing deposits of pale staining amorphous material (amyloid) on H and E stain. H&E = hematoxylin and eosin.

- Aggregates of randomly oriented fibrillary structures on electron microscopy.
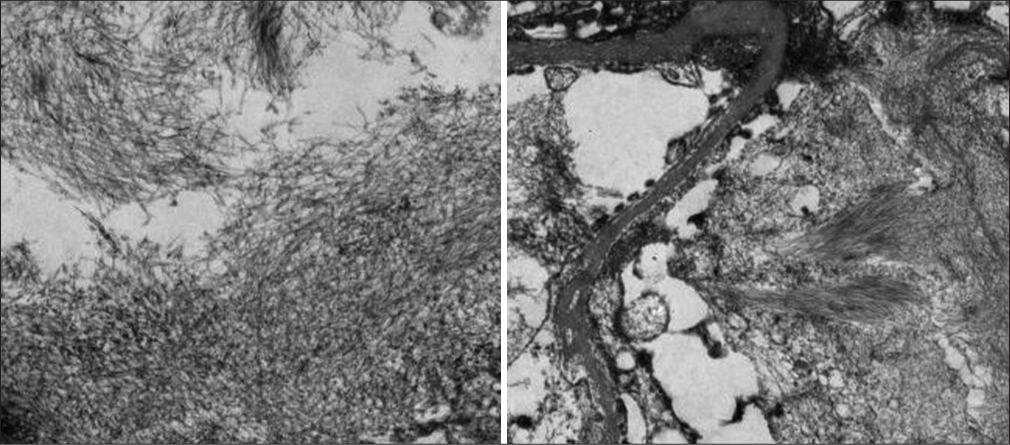
- Markedly expanded mesangial and subendothelial areas occupied by aggregates of randomly oriented, nonbranching fibrillary structures measuring about 9–12 nm in diameter (mean fibril diameter 10.8 nm) with focal “streaming” arrangement. CyBorD = cyclophosphamide, bortezomib, and dexona, GN = glomerulonephritis.
The decision to treat this patient for CLL was difficult as we could not demonstrate the locoregional or hematogenous spread of primary malignancy to the kidneys, and the stage of CLL Rai stage 0 did not mandate treatment. He received six monthly cycles of rituximab and steroids. After treatment, his blood counts normalized, along with a reduction in proteinuria. The serum free light chains also reduced [Table 1].
Case 2
A 70-year-old male, a reformed smoker, with obesity, chronic obstructive pulmonary disease (COPD), systemic hypertension, coronary artery disease (CAD), and Coronary artery bypass graft was evaluated for progressive proteinuric renal dysfunction. Laboratory evaluation showed urine protein creatinine ratio (UPCR)- 1.5, urea- 83 mg/dl, serum creatinine- 1.8 mg/dl, uric acid- 12.9 mg/dl, serum calcium- 9.2, and hemoglobin 11.6 g/dl. NT-Pro BNP was 5910 pg/ml and echocardiogram (ECHO) revealed Type 3 diastolic dysfunction with concentric left ventricular hypertrophy. Renal biopsy showed amyloidosis with kappa restriction. SIFE had elevated free kappa light chain levels and significant elevation of free kappa/lambda ratio [Table 1]. The urine free light chain assay also showed elevated free kappa and lambda chain excretion [Table 2]. Bone marrow examination was normal. He was diagnosed with renal amyloidosis amyloid light chain (AL)-kappa light chain.
| Urine free light chain assay | Patient value | Normal range |
|---|---|---|
| Free kappa light chain | 56.90 mg/l | 1.35-24.19 |
| Free lambda light chain | 11.60 | 0.24-6.66 |
| Free kappa/lambda ratio | 4.91 | 2.04-10.37 |
He received six cycles of cyclophosphamide, bortezomib, and dexona (CyBorD) chemotherapy followed by bortezomib maintenance therapy. Renal functions improved to urea- 58 mg/dl and creatinine- 2.5 mg/dl. Serum free light chain assay normalized after treatment [Table 1].
Case 3
A 66-year-old male with history of hypertrophic non-obstructive cardiomyopathy presented with nephrotic range proteinuria and renal dysfunction. He had UPCR 5.55. Renal biopsy showed 11 glomeruli with mesangial hypercellularity. IF and EM revealed lambda-type amyloidosis [Table 1]. SIFE was positive for “M” band (0.51 g %) in beta 1 region, and serum free light chains were elevated. Bone marrow study was normal. He received treatment with CyBorD regimen. After 4 months of treatment, his UPCR was 1.4 and creatinine was 1 mg/dl. At 6 months, the M band was negative. The lambda free light chain levels had decreased [Table 1].
Case 4
A 51-year-old male with history of hypothyroidism presented with nephrotic range proteinuria. Lab evaluation showed micro-hematuria with UPCR 9.7. Renal biopsy done showed renal amyloidosis-lambda. SIFE reported elevated free lambda levels and a low normal kappa/lambda ratio. Bone marrow examination was normal. He received chemotherapy with daratumab, cyclophosphamide, bortezomib, and dexona. After treatment, the free light chains levels were better, but proteinuria persisted [Table 1].
Discussion
Fibrillary GN, reported to account for 0.5%–1.0% of GN,3 is also a fibril-related disease, and the distinguishing features from amyloidosis are tabulated [Table 3]. Primary hematologic malignancies are known to be associated with glomerular disease during the course of the illness or treatment. Case 1 discussed here is peculiar in that evaluation of the nephrotic syndrome led to the diagnosis of the CLL Rai stage 0. Glomerular lesions are relatively common in lymphoproliferative disorders that lead to plasma cell terminal differentiation and secretion of the M-protein by monoclonal B cells.4 But B-cell malignancy that does not lead to plasma cell differentiation, such as CLL, is less frequently associated with glomerular lesions. The causative link between CLL and GN in the cases reported is unclear.5
| Characteristics | Amyloidosis (AL type) | Fibrillary glomerulonephritis |
|---|---|---|
| Congo red staining | Yes | No (not always) |
| Composition | Fibrils | Fibrils |
| Fibril or | 8-15 nm | 12–22 nm |
| microtubule size | Random | Random |
| Organization in tissues | ||
| Immunoglobulin deposition | Monoclonal LC | Usually polyclonal (mostly IgG4) |
| Glomerular lesions | Deposits spreading from mesangium | MPGN, CGN, MP |
| Renal presentation Extrarenal | Severe NS, absence of hypertension | NS with hematuria, RPGN |
| manifestations | Systemic deposition disease | Pulmonary hemorrhage |
| Association with LPD | Yes (myeloma) | Uncommon |
| Treatment | Melphalan + dexamethasone | Corticosteroid + cyclophosphamide |
AL=amyloid light chain, CGN=crescentic glomerulonephritis, LC=light chain, LPD=lymphoproliferative disorder, MP=mesangial proliferation, MPGN=membranoproliferative glomerulonephritis, NS=nephrotic syndrome, RPGN=rapidly progressive glomerulonephritis
Touchard et al.6 reported the presence of fibrillary structures in two of three cases of nephrotic syndrome associated with CLL. Moulin et al.7 also reported two cases of glomerular fibrillary structures arranged randomly, without cryoglobulinemia and in the absence of circulating M-component. Deletion of 13q14 [del(13q)] is the most common cytogenetic change (50%) in CLL. It is a good prognostic factor if it is detected as a sole aberration by FISH,8 as the case reported here (Case 1).
For elderly patients with history of CAD and renal dysfunction, one would be inclined to consider the etiology as cardiorenal syndrome. The rapidly progressing renal dysfunction, disproportionate proteinuria, alteration in albumin globulin ratio, left ventricular diastolic dysfunction, and elevated levels of NT-proBNP may warrant evaluation for a monotypic clone. Diagnostic efficacy of M band in the traditional serum protein electrophoresis has been outpowered by the quantitative serum protein immunofixation electrophoresis, which includes the free light chain assay and serum immunoglobulin levels along with the protein electrophoresis. The presence of monoclonal production of free light chain will result in an abnormal kappa/lambda ratio.9 None of our patients had bone marrow involvement. All the four patients responded to the treatment targeting to the clone, and the renal functions had returned to normal or stabilised. Case 4 had persistent proteinuria, though the levels of lambda light chains reduced.
Conclusion
Evaluation of abnormal proteinuria and renal dysfunction is mostly complete with SIFE and electron microscopy identifying the abnormal clone. Targeting only renal dysfunction without addressing the clone does not offer adequate results or can even lead to rapid worsening of renal function. Presence of M band, hypercalcemia, anemia, or other components of “CRAB” described in multiple myeloma are not mandatory in suspecting a diagnosis of MGRS. Elevated NT-proBNP, diastolic dysfunction, and peripheral neuropathy are other findings that raise a suspicion of amyloidosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Dysproteinemia and the kidney: Core curriculum 2019. Am J Kidney Dis. 2019;74:822-36.
- [CrossRef] [PubMed] [Google Scholar]
- Monoclonal gammopathy of renal significance. N Engl J Med. 2021;384:1931-41.
- [CrossRef] [PubMed] [Google Scholar]
- New aspects of fibrillary and immunotactoid glomerulonephritis. EMJ Nephrol. 2019;7:78-84.
- [CrossRef] [Google Scholar]
- Fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol. 2008;19:34-7.
- [CrossRef] [PubMed] [Google Scholar]
- Fibrillary glomerulonephritis: An update. Kidney Int Rep. 2019;4:917-22.
- [CrossRef] [PubMed] [Google Scholar]
- Nephrotic syndrome associated with chronic lymphocytic leukemia: An immunological and pathological study. Clin Nephrol. 1989;31:107-16.
- [Google Scholar]
- Glomerulonephritis in chronic lymphocytic leukemia and related B-cell lymphomas. Kidney Int. 1992;42:127-35.
- [CrossRef] [PubMed] [Google Scholar]
- Which prognostic marker is responsible for the clinical heterogeneity in CLL with 13q deletion? Mol Cytogenet. 2021;14(2)
- [CrossRef] [PubMed] [Google Scholar]
- Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812-7.
- [CrossRef] [PubMed] [Google Scholar]







