Translate this page into:
Mortality and Low Serum Bicarbonate Level in Patients on Hemodiafiltration versus Peritoneal Dialysis
Address for correspondence: Dr. V. D. Raikou, 17, Agiou Thoma, Athens, Greece. E-mail: vraikou@med.uoa.gr
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Mortality is substantially elevated in patients on chronic kidney disease in comparison to general population. In this study, we observed the mortality rate in relation to risk factors including low serum bicarbonate level, coronary artery disease (CAD), and dialysis modality in patients on dialysis during a median follow-up time of 60 months. We studied 96 dialysis patients, 62 males and 34 females, on mean age 62.1 ± 14.27 years old. The treatment modalities which were applied were predilution hemodiafiltration (HDF, n = 76), and peritoneal dialysis (PD, n = 20). We performed Kaplan–Meier curves and a Cox-regression analysis to investigate significant risk factors for mortality including hypertension, diabetes mellitus, smoking, bone disease defined by intact-parathormone, serum albumin, serum bicarbonate levels < or >22 mEq/L, dialysis modality, and the existence of CAD. Cox-regression analysis revealed a significant impact of serum bicarbonate levels <22 mEq/L on mortality in combination to dialysis modality and CAD. The prevalence of CAD on mortality was found significant (log-rank = 5.507, P = 0.02). Furthermore, the impact of dialysis modality on mortality was shown significant (log rank = 22.4, P = 0.001), noting that during the first 28–30 months from the treatment initiation, the survival was better for PD; but then, the mortality was significantly increased comparatively to HDF. Uncorrected metabolic acidosis and CAD were shown as independent significant predictors for mortality in patients on renal replacement therapy. PD may provide worse survival after 2–2.5 years of treatment initiation than HDF.
Keywords
Coronary artery disease
hemodiafiltration
metabolic acidosis
peritoneal dialysis
survival
Introduction
Mortality is potentially elevated in patients on chronic kidney disease (CKD) in comparison to general population. Previously, it has been showed that patients in CKD stages 4 and 5 approached a rate of three-fold and six-fold higher mortality risk, respectively, than patients with glomerular filtration rate (GFR) >60 ml/min/1.73 m2.[12]
Dialysis mortality was shown as an eight-fold higher age-standardized mortality compared to general population unseparated to dialysis modality.[3] However, it has been reported that mortality may differ between dialysis modality. Comparative studies between patients treated with peritoneal dialysis (PD) and hemodialysis (HD) have frequently shown conflicting results.[4] It has been shown that PD patients have a higher survival rate depended on dialysis vintage for younger and nondiabetic patients than HD patients despite in some studies, HD displayed better survival.[5] Controversially, previous study from a single Chinese center showed that dialysis modality itself has no effect on the survival rate of dialysis patients.[6]
The cardiovascular disease is recognized as the main reason for the increased mortality in dialysis patients.[7] Recently, it has been suggested that the unfavorable effects of metabolic acidosis including malnutrition, inflammation, and oxidative stress can contribute to elevated mortality in dialysis patients.[89]
In this study, we observed the mortality rate in relation to risk factors including low serum bicarbonate level, coronary artery disease (CAD), and dialysis modality in patients on dialysis during a median follow-up time of 60 months.
Methods
Patients
This is a retrospective study of a cohort of 96 dialysis patients, 62 males and 34 females, on mean age 62.1 ± 14.27 years old. The data was collected during a time of 60 months, from the 1st of January 2007 until the end of December 2011.
The treatment modalities which were applied were online predilution hemodiafiltration (HDF, n = 76) and PD (n = 20). The median time on HD was 5.0 ± interquartile range 3–10 years and the mean time on PD was 2.8 ± 1.61 years before the starting of our study. In our data, 13 patients were initiated dialysis treatment after the starting of this study, and 83 patients were already in permanent dialysis therapy.
We excluded patients <18 years of age at initiation of dialysis and patients who had <6 months of follow-up. Patients without regular vascular HD access and who had dialysis catheter and those with autoimmune diseases, infections, or malignancy were excluded from our study. Particularly for the enrolled patients on PD, those who had been on HD or received a kidney transplant before the initiation of PD and patients who started PD for other reasons, such as congestive heart failure or acute renal failure, were excluded from the study.
The HD treatment was performed three-times weekly with a dialysis time of 3.5–4 h per session, a filter of 1.5–2 m2 surface area by high-flux synthetic membrane, defined by a ultrafiltration coefficient >20 ml/h,[10] and a blood flow of 350–400 ml/min. A bicarbonate-based ultrapure buffer dialysis solution was used with a dialysate flow rate of 500–600 ml/min, a calcium concentration of 1.50–1.75 mmol/L, a sodium concentration of 138–145 mmol/L, and low molecular weight heparin as anticoagulant therapy. The final concentration of bicarbonate in dialysate was 32 mEq/L. Dialysis dose was defined by Kt/V/day for urea which was calculated according to the formula of Daugirdas.[11] Patients were excluded if they had Kt/V for urea <1.2.
The included PD patients were following continuous ambulatory PD with 4 changes/day using a combination of 2 changes of 2 L of hypertonic glucose-based solution (3.86% glucose; Baxter Healthcare) and 2 changes of 2 L of semihypertonic glucose solution (2.5% glucose; Ariti; Bieffe Medital S.P.A). All patients underwent urea kitenic analysis including residual renal function every 3 months of PD initiation. Dialysis dose defined according to the formula of Daugirdas by total Kt/V/week for urea including peritoneal Kt/V urea and residual GFR (ml/min/1.73 m2). The patients who had Kt/V/week for urea <1.7 were excluded from our study. We used peritoneal liquids in dual backs with a final concentration of bicarbonate equal to 37.5 mEq/L.
The enrolled patients were in a good status, they did not have interdialytic peripheral edema, high blood pressure, interdialytic orthostatic hypotension, or other characteristics of an inaccurate dry body weight. However, patients with predialysis blood pressure ≥140/90 (n = 40, a ratio of 41.7%) were considered hypertensive or if they were receiving antihypertensive drugs. Twenty-one of the studied patients were current smokers (a ratio of 21.9%).
At start of the study, the existence of coronary syndrome (n = 30, a ratio of 31.3%) was documented by history of myocardial infarction, coronary artery angioplasty or bypass surgery, or clinical signs of angina pectoris. Furthermore, the first and the current cardiovascular events during the study were written down as one event for the CAD manifestation.
Twenty HD patients and 15 PD patients excreted up to 100 ml of urine/day. Calcium channel blockers, beta-blockers, or inhibitors of angiotensin II receptors were included in the receiving medications by our patients. Some of the enrolled patients were receiving statin, folic acid, and only calcium-free phosphate binders were prescribed. All of the studied patients were on erythropoietin-α or -β therapy.
The underlying renal diseases were hypertensive nephrosclerosis (n = 31), chronic glomerulonephritis (n = 28), polycystic kidney disease (n = 12), diabetic nephropathy (n = 11), and other/unknown (n = 14).
Approval and consent
The study was approved by the Ethics Committee of the hospitals “Laiko, University General Hospital of Athens” and Renal Unit of “Diagnostic and Therapeutic Center of Athens Hygeia SA.” Written informed consent was obtained from all subjects.
Blood collection
Blood samples were obtained by venipuncture in the PD patients in a 12 h fasting state during an regular appointment in our peritoneal unit. In HD patients, blood was drawn just before the start of the mean weekly dialysis session also in a 12 h fasting state from the vascular access. In the end of the treatment, the blood pump speed was reduced to <80 ml/min and blood samples was obtained at 2 min postdialysis from the arterial dialysis tubing for the calculation of the adequacy of dialysis by Kt/V for urea.
Samples were centrifuged immediately; serum was separated and processed for various assays.
In each subject, three sequences of samples (every month within 3 months) were received for the serum bicarbonate measurements, and their average was used for statistical analysis.
Laboratory measurements
Albumin, high-density lipoproteins (HDLs), and low-density lipoproteins (LDLs) were measured by biochemical analysis and the ratio of LDL/HDL was calculated.
High-sensitivity C-reactive protein (hsCRP) and oxidized LDL (ox-LDL) serum concentrations were measured using enzyme-linked immunosorbent assays (Immundiagnostik AG, Germany and Immundiagnostik AG. Stubenwald-Allee, Bensheim, respectively) according to manufacturer's specifications.
The concentrations of intact parathormone (i-PTH) and beta-2-microglobulin (beta2M) were measured by radioimmunoassays (CIS bio International/France and Immunotech by Beckman, Czech Republic respectively).
Insulin levels were measured using a immunoradiometric assay (BioSource Europe SA, Belgium) with a reported interassay coefficient of variation 6.1%.
Insulin resistance (IR) was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR).[12]
Metabolic acidosis was defined by serum bicarbonate concentrations <22 mEq/L, which were measured in gas machine (Roche, cobas b 121) taking care of the blood specimens.[13] The low serum bicarbonate level was considered in combination to low arterial pH (acidemia) and decreased PCO2, thus the decreased serum bicarbonate concentrations to reflect metabolic acidosis rather than respiratory alkalosis. Respiratory alkalosis is another clinical condition that causes decreased bicarbonate level in the end-stage of renal disease (ESRD) patients, due to the loss of buffering capacity by the kidney in these patients.
Normalized protein catabolic rate for dry body mass was calculated from the urea generation rate.[14] Body mass index (BMI) was obtained from height and postdialysis body weight.
Hemodynamic measurements
Predialysis peripheral systolic blood pressure and diastolic blood pressures (SBP and DBP, respectively) in enrolled patients were calculated as the mean of 10 measurements during a treatment month using an automatic sphygmomanometer OMRON M4-I (Co., Ltd. Kyoto, Japan). Mean peripheral predialysis BP (MBP) was calculated as MBP = DBP + 1/3 (SBP − DBP).
Electrocardiographical analysis and M-mode echocardiography were performed the day after dialysis with an Hewlett Packard, SONOS 2500 using a 2.25 MHz transducer to estimate the ejection fraction and the ischemic findings according to the recommendations of the American Society of Echocardiography.[15]
Arterial stiffness was measured as carotid-femoral pulse wave velocity (c-f PWV) and carotid augmentation index (AIx) using the SphygmoCor System® (AtCor Medical Pty. Ltd, Sydney, Australia). In each subject, two sequences of measurements were performed, and their mean was used for statistical analysis. We recorded the c-f PWV by positioning one sensor over the right femoral artery and a second sensor over the left carotid artery. The distance between the two sensors was measured with a measuring tape, and three recordings of both pulse waveforms were performed (8–10 heart beats for each recording). The Complior software (Colson, Garges les Genosse, France, Software version 2.1) automatically detected the foot of each pulse waveform from the two arterial sites and then measured the mean distance between the two feet as being the travel time of the wave. PWV was then computed using the formula: PWV = travel distance/travel time as previously validated.[16]
Central SBP, DBP, MBP, pulse pressure (PP), and the time of return of the reflected wave (Tr) were derived. Pressure and time of first peak (P1 and T1) and second peak (P2 and T2) and central augmented pressure (AP) were obtained. Central AIx was computed (AP = P2 − P1; AIx = [AP/PP] × 100) and corrected for a heart rate of 75 beats/min.
Data analysis
Data were analyzed using SPSS version 15.0 statistical package for Windows (SPSS Inc., Chicago, Illinois, USA) and expressed as mean ± standard deviation or as median value (interquartile range) for data that showed skewed distribution; differences between mean values were assessed using unpaired t-test for two groups and data that showed skewed distributions were compared with Mann–Whitney U-test.
Correlations between variables were defined by Pearson and Spearman coefficient and P < 0.05 was considered statistically significant. Correlations between categorical variables were defined by log-rank tests with Kaplan–Meier analysis during our median follow-up time of 60 months. The total dialysis vintage, determined from the starting of dialysis treatment until the end of the follow-up of 60 months of our study, was used for the definition of mortality rate in our data by life table and also for the prevalence of dialysis modality on mortality by Kaplan–Meier curve. We performed a Cox-regression analysis to investigate significant risk factors for mortality including traditional and specific confounders for these patients, such as hypertension, diabetes mellitus, smoking, bone disease defined by i-PTH, serum albumin, serum bicarbonate levels < or >22 mEq/L, dialysis modality, and the existence of CAD.
Results
Demographical characteristics of the studied population at the time of inclusion are listed in Table 1.

In this study, 30 patients died from the starting of treatment vintage until the end of our follow-up time of 60 months [a mortality rate equal to 31.2%, survival function in Figure 1]. The main reasons of death were cardiovascular events (n = 22, a ratio of 73.3%), sepsis (n = 5, a ratio of 16.7%), and other (n = 3, a ratio of 10%).
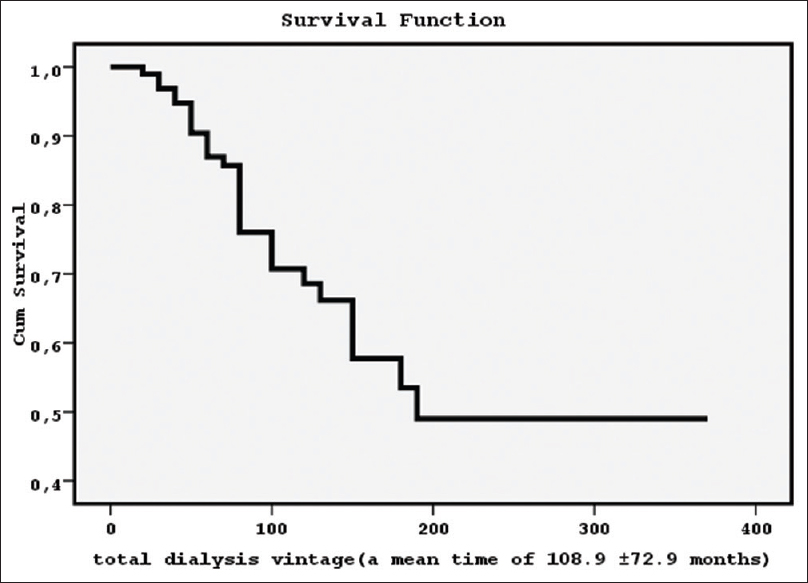
- The survival function for 96 dialysis patients from the treatment initiation until the end of our follow-up of 60 months (a mean time of 108.9 ± 72.9 months) showed a mortality rate equal to 31.2%
Kaplan–Meier analysis for the prevalence of CAD on mortality was found significant [log-rank = 5.507, P = 0.02, hazard function in Figure 2]. Furthermore, the impact of dialysis modality on mortality was shown statistically significant [log-rank = 22.4, P = 0.001, hazard function in Figure 3] using the total dialysis time for our data since the starting of treatment vintage until the end of our follow-up time of 60 months. Particularly, we noted that during the first 28–30 months from treatment initiation, the survival was better for PD; but then, the mortality was significantly increased comparatively to the patients on HDF. However, the relationship between dialysis modality and the existence of CAD by Kaplan–Meier analysis was found nonsignificant. The impact of serum bicarbonate levels < or >22 mEq/L on mortality was found nonsignificant. In addition, the relationship between serum bicarbonate levels < or >22 mEq/L and both, existence of CAD or dialysis modality was found nonsignificant.
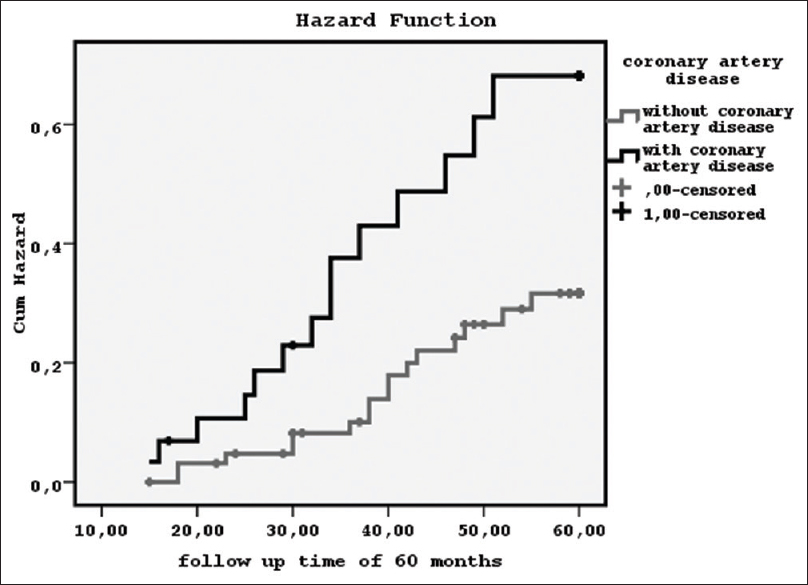
- The prevalence of the existence of coronary artery disease on mortality during a follow-up time of 60 months by Kaplan–Meier curve (log-rank = 5.507, P = 0.02)
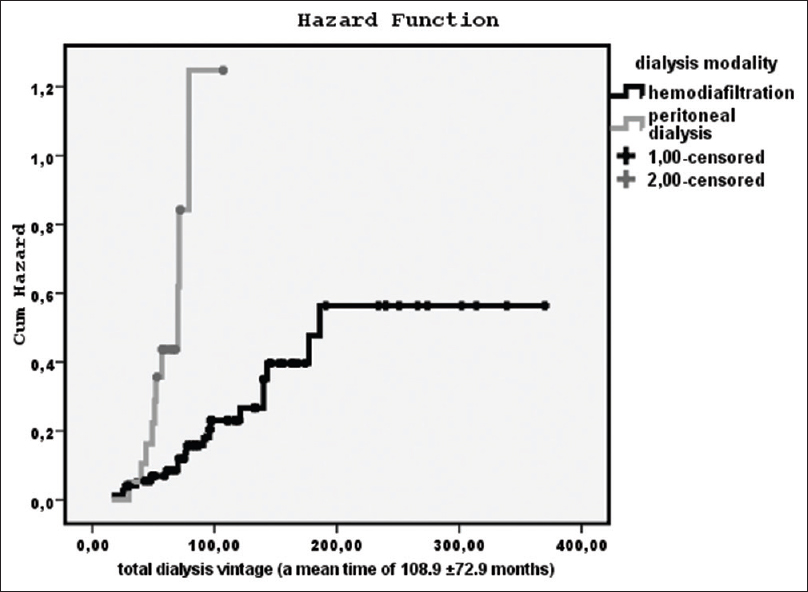
- The impact of dialysis modality on mortality from the treatment initiation until the end of our follow-up of 60 months (a mean time of 108.9 ± 72.9 months) by Kaplan–Meier curve (log-rank = 22.4, P = 0.001)
Nevertheless, Cox-regression analysis revealed a significant impact of serum bicarbonate levels < or >22 mEq/L on mortality in combination to dialysis modality and CAD [Table 2] adjusting for hypertension, diabetes mellitus, smoking, bone disease defined by i-PTH, and serum albumin.

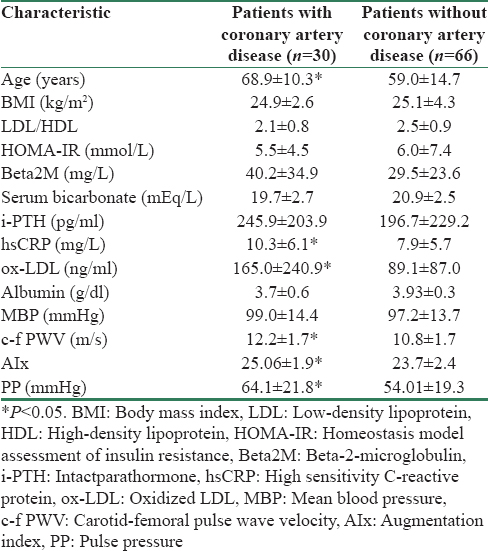
In Table 3, the differences between the groups of patients with CAD manifestation or without CAD are shown. We observed that the patients with CAD had higher age, beta2M, i-PTH, c-f PWV, PP, AIx, ox-LDL, and hsCRP but lower serum bicarbonate concentrations, BMI, and albumin than the patients without manifested CAD.
In Table 4, the differences between the groups of patients according to dialysis modality are shown. We noted that the patients on HDF had lower i-PTH, serum bicarbonate levels, hsCRP, IR defined by HOMA-IR but higher albumin, ox-LDL, and beta2M than the patients on PD.

In Table 5, the differences between the patients with serum bicarbonate levels < or >22 mEq/L are shown. We observed that the patients with serum bicarbonate levels <22 mEq/L were older, they had higher beta2M, HOMA-IR, i-PTH, hsCRP, ox-LDL, c-f PWV, PP, and AIx, but lower albumin level and lower urine volume in comparison to the patients with serum bicarbonate levels >22 mEq/L.

Discussion
In this study, we observed that the patients with manifested CAD had greater mortality rate than the patients without CAD and the existence of CAD was found such as a significant independent predictor for mortality adjusting for potentially multiple confounding covariates. Furthermore, the patients with manifested CAD had higher c-f PWV, Aix, and PP comparatively to the patients without CAD.
Increased PWV and AIx are integrated indexes of vascular function and structure. They estimate the arterial stiffness, which is a strong predictor of cardiovascular mortality in general population and in dialysis patients.[17] Furthermore, elevated PP is one another consequence of arterial stiffening and vascular calcification. Previously, it has been shown the positive relationship between the extent of vascular calcification and arterial stiffness and it may explain the increased cardiovascular events seen in dialysis patients.[18]
In addition, it has been already established that ESRD results in accelerated atherosclerosis and increased morbidity and mortality.[7] Even when the patients are undergoing renal replacement therapy, the mortality remains high, mainly for cardiovascular causes due either to uremia-related risk factors (such as anemia, hyperparathyroidism, inflammation, oxidative stress, and malnutrition)[1920] or to traditional ones (age, male gender, diabetes, obesity, hypertension, smoking, dyslipidemia).[2122] Indeed, in the present study, the patients with manifested CAD were older and they had higher i-PTH, hsCRP, ox-LDL, beta2M, and worse status of metabolic acidosis in combination to lower albumin and BMI, which are some of the malnutrition characteristics, than the patients without CAD.
Interestingly, in our study, even though Cox-regression analysis revealed significant impact of low serum bicarbonate levels on mortality adjusting for potentially multiple confounders, Kaplan–Meier analysis did not show a significant prevalence of low serum bicarbonate levels on mortality rate, neither on the existence of CAD. These findings may suggest that metabolic acidosis defined by low serum bicarbonate level may be an independent predictor for mortality in relation to confounders in dialysis patients.
In addition, in this study, we observed that the patients with low bicarbonate level (<22 mEq/L) had higher c-f PWV, AIx, PP, beta2M, IR defined by increased HOMA-IR, i-PTH, hsCRP, ox-LDL, but lower albumin level and lower urine volume as an indicator of decreased residual renal function, than the patients with serum bicarbonate levels >22 mEq/L.
These findings support that metabolic acidosis results in detrimental effects and patients with low bicarbonate level should be treated properly even though they are receiving dialysis therapy. Previously, it has already been reported the role of metabolic acidosis on vascular calcification as the mineral metabolism disturbances act through the existing metabolic acidosis in dialysis patients.[23] The influence of acidosis on vascular calcification is complicated, acting as a stimulator of the solubility of Ca xPdeposits and as a blocker of phosphate uptake by the arterial smooth muscle cells, so acidosis may attenuate vascular calcification.[23] However, on the other hand, acidosis promotes inflammation of the arterial wall, releasing cytokines that may induce vascular calcification.[24] In addition, preliminary evidence suggested that metabolic acidosis may play a role in the accumulation of beta2M, which serves as a surrogate marker of middle molecules' uremic toxins and in the hypertriglyceridemia seen in hemodialyzed patients.[2526] Previously, it has been already demonstrated the role of beta2M on mortality in hemodialyzed patients, independently hemodialysis duration, diabetes, and malnutrition,[27] due mainly to vascular inflammation and amyloid formation in the vessel wall resulting in vessels damage.[28]
In the meantime, there is no consensus about the optimal bicarbonate levels in ESRD patients. A few studies have addressed this issue and they have conflicting results.[929] Previous study showed that serum bicarbonate level >22 mEq/L was associated with lower mortality risk[30] and another study reported that an increased risk was observed in patients with high (>27 mEq/L) or low (<17 mEq/L) bicarbonate levels.[31] In this study, we were considered uncorrected metabolic acidosis in enrolled patients, when serum bicarbonate level was <22 mEq/L combined to low arterial pH and decreased PCO2. Therefore, the low bicarbonate level may be diagnostic of metabolic acidosis rather than of respiratory alkalosis, another clinical condition that causes decreased bicarbonate level, given the loss of buffering capacity by the kidney in ESRD. However, in our data, the mean value of serum bicarbonate concentrations, particularly for enrolled patients on HDF, was lower comparatively to previous reports,[31] due may to the differently used bicarbonate concentration in dialysis dialysate. Furthermore, we did not exclude from the study the diabetic patients who may have worse metabolic acidosis state in our baseline measurements.
In agreement to our results, the underlying pathophysiological mechanisms for increased mortality and morbidity in dialysis patients with low serum bicarbonate levels include metabolic disorders, bone disease, chronic inflammation,[32] and loss of residual renal function that has been established to be a powerful predictor of mortality in patients on dialysis.[33]
Moreover, in this study, the dialysis modality showed a significant influence on mortality rate notifying that the patients on PD presented worse survival than the patients on HDF despite dialysis modality was not significantly associated with the existence of CAD, neither with low bicarbonate level by Kaplan–Meier curves during our follow-up time of 60 months. Specifically, during the first 28–30 months from treatment initiation, the survival was better for PD; but then, the mortality was significantly increased comparatively to the patients on HDF. Supportingly, Cox-regression analysis showed dialysis modality to be an important predictor for mortality adjusting for confounders. Comparing the patients on HDF and on PD between them, we observed that the patients on HDF had lower i-PTH, HOMA-IR, serum bicarbonate concentrations, BMI, and hsCRP, but higher beta2M, albumin, and ox-LDL than the patients on PD. According to our findings, despite the continuous provision of PD treatment, the patients on PD had more metabolic disturbances, malnutrition, excited inflammation, and bone disease, but better metabolic acidosis status, may lower oxidative stress and relatively decreased beta2M serum concentrations than the patients on HDF. These factors should be connected to the pathophysiological mechanisms which contribute to the different survival rate between HDF and PD.
Comparative studies between patients treated with PD and HD have frequently provided conflicting results, due may to methodological and design differences.[3034] It has been reported that PD patients have a slightly higher survival rate in the first 1–2 years, in agreement with our findings.[35] Other study found that PD patients had better survival irrespectively to treatment vintage.[36] In contrast, in some studies, HD displayed better survival or both dialysis modalities had similar effect on survival rate.[56]
There are several limitations in the present study. This is an observational study with a relatively small sample size. However, we tried to enrol patients in a good status minimizing the risk of inclusion of more sick patients with excited cardiovascular disease or probably connected to serum bicarbonate <22 mEq/L. Furthermore, data representing overall nutritional status assessment, anthropometry, and dietary protein intake were not available for the analysis.
Conclusion
Uncorrected metabolic acidosis and CAD were shown as independent significant predictors for mortality in patients on renal replacement therapy. PD may provide worse survival after 2–2.5 years of treatment initiation than HDF.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-305.
- [Google Scholar]
- The natural history of chronic renal failure: Results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis. 2005;46:863-70.
- [Google Scholar]
- Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302:1782-9.
- [Google Scholar]
- Mortality studies comparing peritoneal dialysis and hemodialysis: What do they tell us? Kidney Int Suppl. 2006;103:S3-11.
- [Google Scholar]
- Comparison of patient survival between hemodialysis and peritoneal dialysis in a single Chinese center. Int Urol Nephrol. 2014;46:2403-7.
- [Google Scholar]
- Cardiovascular disease in the dialysis population: Prognostic significance of arterial disorders. Curr Opin Nephrol Hypertens. 2006;15:105-10.
- [Google Scholar]
- Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin Dial. 2004;17:455-65.
- [Google Scholar]
- A low serum bicarbonate concentration as a risk factor for mortality in peritoneal dialysis patients. PLoS One. 2013;8:e82912.
- [Google Scholar]
- Dialyzer membrane permeability and survival in hemodialysis patients. Am J Kidney Dis. 2005;45:565-71.
- [Google Scholar]
- Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J Am Soc Nephrol. 1993;4:1205-13.
- [Google Scholar]
- Impact of abdominal fat and insulin resistance on arterial hypertension in non-obese women. Arq Bras Endocrinol Metabol. 2009;53:340-3.
- [Google Scholar]
- Spurious metabolic acidosis in hemodialysis patients. Am J Kidney Dis. 2000;35:1068-71.
- [Google Scholar]
- Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv Ren Replace Ther. 1995;2:295-304.
- [Google Scholar]
- Plasma concentration of brain natriuretic peptide as an indicator of cardiac ventricular function in patients on hemodialysis. Am J Nephrol. 1998;18:411-5.
- [Google Scholar]
- Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485-90.
- [Google Scholar]
- Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434-8.
- [Google Scholar]
- Association of pulse wave velocity with vascular and valvular calcification in hemodialysis patients. Kidney Int. 2007;71:802-7.
- [Google Scholar]
- Effects of native and modified low-density lipoproteins on monocyte recruitment in atherosclerosis. Hypertension. 2007;50:276-83.
- [Google Scholar]
- All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173-82.
- [Google Scholar]
- Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899-911.
- [Google Scholar]
- Vascular calcification and metabolic acidosis in end stage renal disease. Hippokratia. 2009;13:139-40.
- [Google Scholar]
- Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int. 1997;51:1678-95.
- [Google Scholar]
- Potential effect of metabolic acidosis on beta 2-microglobulin generation: In vivo and in vitro studies. J Am Soc Nephrol. 1996;7:350-6.
- [Google Scholar]
- Effect of metabolic acidosis on hyperlipidemia in uremia. Pediatr Nephrol. 1999;13:891-3.
- [Google Scholar]
- Serum beta2-microglobulin level is a significant predictor of mortality in maintenance haemodialysis patients. Nephrol Dial Transplant. 2009;24:571-7.
- [Google Scholar]
- Beta2-microglobulin as a biomarker in peripheral arterial disease: Proteomic profiling and clinical studies. Circulation. 2007;116:1396-403.
- [Google Scholar]
- Dialysis modality and correction of uremic metabolic acidosis: Relationship with all-cause and cause-specific mortality. Clin J Am Soc Nephrol. 2013;8:254-64.
- [Google Scholar]
- Association between serum bicarbonate and death in hemodialysis patients: Is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006;1:70-8.
- [Google Scholar]
- Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44:661-71.
- [Google Scholar]
- Cytokine secretion and markers of inflammation in relation to acidosis among chronic hemodialysis patients. Blood Purif. 2013;35:181-6.
- [Google Scholar]
- Emerging importance of residual renal function in end-stage renal failure. Semin Dial. 2011;24:487-94.
- [Google Scholar]
- Comparing the risk for death with peritoneal dialysis and hemodialysis in a national cohort of patients with chronic kidney disease. Ann Intern Med. 2005;143:174-83.
- [Google Scholar]
- Hemodialysis and peritoneal dialysis: Comparison of adjusted mortality rates according to the duration of dialysis: Analysis of The Netherlands Cooperative Study on the Adequacy of Dialysis 2. J Am Soc Nephrol. 2003;14:2851-60.
- [Google Scholar]
- Mortality in chronic kidney disease and renal replacement therapy: A population-based cohort study. BMJ Open. 2014;4:e004251.
- [Google Scholar]







