Translate this page into:
Nephrocalcinosis—A gateway to the Diagnosis
Address for correspondence: Dr. Agrata Sharma, Senior Resident (DM)- Neurology, AIIMS, Bhopal, Madhya Pradesh, India. E-mail: dragrata@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Nephrocalcinosis (NC) is the augmented calcium content within the renal parenchyma. Its pathogenesis mainly involves hypercalciuria. The presence of medullary NC provides a window to the clinician for the diagnosis of many important diseases. In this case series, we highlight three diseases that could be diagnosed with a high index of suspicion and detailed evaluation after their presentation as medullary NC.
Keywords
Adrenal adenoma
distal renal tubular acidosis
nephrocalcinosis
parathyroid adenoma
Sjogren's syndrome
Introduction
Nephrocalcinosis (NC) is augmented calcium content within the renal parenchyma.[1] When it is in the pyramids of kidneys it is called medullary NC (MNC), it is commoner than cortical and could be explained by the location of the major homeostatic pathway of calcium reabsorption and the segments linked to acid-base regulation in the medulla.[2] Conditions that trigger hypercalcemia, hyperphosphatemia, as well as conditions that cause high urinary excretion of calcium, phosphate, and/or oxalate and low urinary citrate can lead to MNC.[1] MNC is an insidious process which in most cases is detected only on incidental radiological investigation. It may be the presenting manifestation of varied disorders and only an in-depth workup can assist in reaching the diagnosis. In the text to follow, we present a series of three unique cases who had MNC in common, who on further detailed evaluation revealed the presence of three different disease conditions.
Case Series
Case 1
A 34-year-old male farmer was admitted to the neurology department with complaints of lower limb weakness and inability to walk for the last 8 months. Weakness was insidious and progressive; affecting proximal muscles as well. His past medical history included repeated hospital admissions for episodes of weakness and fatigue linked with hypokalemia over the last 4 years that were managed as hypokalemic periodic paralysis and responded to supplemental potassium alone. There was no bowel bladder and sensory impairment.
On physical examination, vitals were within normal limits and higher mental functions were intact; with unremarkable cranial nerves. Motor power was 3/5 in proximal upper and lower limbs. Deep tendon reflexes were diminished bilaterally with flexor plantar response. There was no sensory deficit. Cardiovascular, respiratory, gastrointestinal, and thyroid examination findings were normal.
He was found to have hypokalemia with high transtubular potassium gradient (TTKG), normal anion gap acidosis, and mild hyperchloremia. A high urine pH in the presence of metabolic acidosis suggested distal renal tubular acidosis (dRTA). Plain X-Ray of the abdomen showed bilateral MNC [Figure 1a]. The renal USG showed hyperechoic regions in the renal medulla of MNC. The patient was further evaluated for dRTA along with its associated autoimmune disorders.
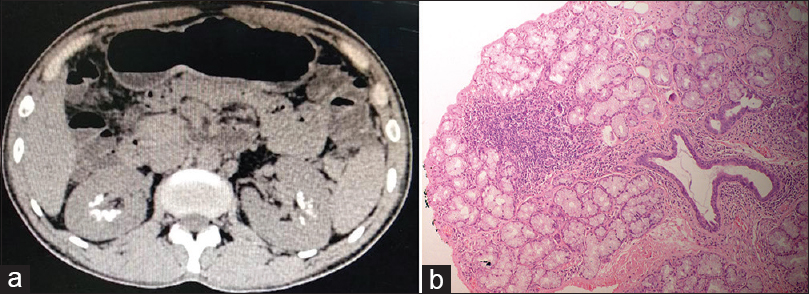
- (a) CT of the abdomen showing bilateral medullary nephrocalcinosis (MNC) and (b) Lip biopsy showing multiple foci of lymphoid aggregates (50 cells/foci) in 2–3 lobules suggestive of Chisholm and Mason grade 4 Sjogren syndrome
Reevaluation of history revealed xerostomia without xerophthalmia and easy and early loosening of teeth. Oral cavity evaluation showed poor dental hygiene with multiple loosening of teeth with an overall dry cavity. Primary Sjogren syndrome (pSS) was suspected, which was later confirmed by significantly raised anti-Ro and anti-La antibodies. Schirmer's test was negative. Lip biopsy [Figure 1b] revealed multiple foci of lymphoid aggregates (50 cells/foci) in 2–3 lobules suggestive of Chisholm and mason grade 4 SS. The patient did not have any associated rheumatic disease and diagnosis of pSS was held. Among other autoimmune disorders, thyroid function tests were deranged with findings suggestive of hypothyroidism with a significant increase inanti-thyroid peroxidase (TPO) revealing Hashimoto thyroiditis with Sjogren.
Diagnosis of dRTA with associated MNC secondary to pSS with Hashimoto thyroiditis was made. Patient was started on potassium supplementation (K citrate) with hydrochlorothiazide for future prevention of stone formation along with immunosuppressants. There was a gradual improvement in serum potassium with normalization of serum pH and bicarbonate levels. He is under regular follow-up. During the last 6 months after discharge, he has not suffered any episodes of weakness and had normal serum potassium, bicarbonate, andpH values with medications including azathioprine (2 mg/kg/day), potassium citrate, and hydrochlorothiazide.
Case 2
A 23-year-old male presented to the department of nephrology with colicky pain in the right lumbar region for the last 3 days radiating to groin and right testes and pain over left scapula and dorsal spine for last 3 months. Past history was significant for recurrent abdomen pain, the passage of stones in urine since the age of 10 years and poor growth as compared to other children in the local vicinity. No family history of renal calculus disease was found. Physical examination was normal except for frail built with a weight of 40 kg and a height of 150 cm.
An emergency X-ray kidney, ureter, and bladder (KUB) and abdomen ultrasonography (USG Figure 2a) was done which revealed right lower ureteric calculus of 6.2 mm, right hydroureteronephrosis, and bilateral MNC and parenchymal disease in both the kidneys. He had chronic kidney disease (CKD) IIIa (e GFR of 50 mL/mt/1.73 m2). He was started on symptomatic treatment for renal colic. A suspicion for RTA type-1 with MNC causing CKD was raised in view of poor growth and recurrent lithuria and workup started. Arterial-blood gas (ABG) confirmed hyperchloremic metabolic acidosis and normal serum anion gap. Urine chemistry showed a positive anion gap, hypercalciuria [Table 1], and normal 24 h oxalate and uric acid excretion. This confirmed type-1 RTA but he had very high serum calcium and low serum phosphorus this led to suspicion of hyperparathyroidism (HPT). A serum iPTH was found to be raised. It confirmed primary HPT as a cause for MNC and recurrent nephrolithiasis with type-1 RTA. USG neck showed a parathyroid gland adenoma, functional status was confirmed by 99mTC myocardial perfusion imaging (MIBI) scan [Figure 2b]. An MRI dorsal spine revealed a D1 expansile lesion, with sclerotic rim and mild heterogeneous contrast enhancement suggestive of Brown tumor. He underwent parathyroid adenomectomy. 1-month post-surgery, his serum calcium, phosphorus, and iPTH levels were 9.03mg/dL, 3.0 mg/dL, and 62 pg/mL with normal urinary calcium excretion with urine calcium to creatinine ratio of 0.06.
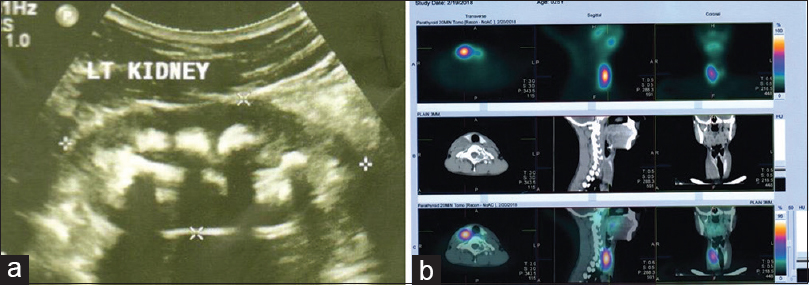
- (a) Ultrasound showing bilateral medullary nephrocalcinosis (MNC) and (b) 99mTC myocardial perfusion imaging (MIBI) scan showing parathyroid adenoma
| Test | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Hb (g/Dl) | 12.3 | 13.4 | 12.2 |
| TLC (per cubic mm) | 4490 | 6200 | 13240 |
| Platelet counts per cubic mm | 2 | 2.5 | 2.37 |
| ESR (mm/h) | 20 | 34 | 24 |
| Serum Na+ (mEq/L) | 139 | 136 | 149 |
| Serum K+ (mEq/L) | 2.31 | 4 | 2.10 |
| Serum chloride (mEq/L) | 118 | 113 | 106 |
| Serum Urea (mg/dL) | 23.49 | 26 | 29 |
| Sr Creatinine (mg/Dl) | 1.20 | 1.85 | 1.22 |
| Sr uric acid (mg/dL) | 2.76* | 8.20 | 5.7 |
| Serum calcium (mg/dL) | 8.29 | 13.24 | 9.20 |
| Sr phosphorus (mg/dL) | - | 2.39 | 2.91 |
| Sr magnesium (mg/dL) | - | - | 2.08 |
| Serum pH | 7.25 | 7.32 | 7.50 |
| pCO2 (mmHg) | 41 | 32 | 42 |
| HCO (m Eq/L) | 20 | 16.6 | 25.2 |
| pO2 (mmHg) | 130 | 200 | |
| Anion gap | 11.2 | 8.7 | |
| Sr Vit | - | 33 | -- |
| D (ng/mL) | |||
| PTH (pg/mL) | - | 1142 | - |
| Sr aldosterone (ng/dL) | - | - | 83.70 |
| Sr reninng/mL/h | - | - | 1.30 |
| Sr aldosterone | - | - | 64.30 |
| Renin ratio | |||
| TSH (mIU/L) | 9 | 4.42 | - |
| Urine pH | 7 | 6 | |
| Urine anion gap (mEq/L) | Positive | Positive | |
| Urine Ca/Creatinine ratio | High | High | High |
| HIV, HBsAg, HCV | Negative | Negative | |
| ANAs by IFA >1:160 | Positive | Negative | |
| CECT Abdomen | - | - | Adrenal adenoma |
| 99mTC | - | Functional | - |
| MIBI scan | Parathyroid adenoma |
*normal range = 3.1 - 7 mg/dL
Case 3
A 42-year-old hypertensive female was admitted to the department of nephrology in view of generalized weakness and fatigability for 3 weeks. On physical examination, vitals were within normal limits except for a high BP of 176/100 mmHg on amlodipine 10 mg/day. Motor power was 4/5 in proximal upper and lower limbs. Deep tendon reflexes were diminished bilaterally with flexor plantar response. Higher mental functions were intact. Cranial nerve examination as within normal limits. There was no sensory deficit. Cardiovascular, respiratory, gastrointestinal, and thyroid examination findings were normal.
A previous USG abdomen revealed bilateral MNC. Further investigations showed renal dysfunction with an eGFR of 53 mL/mt/1.73 m2, hypernatremia, and hypokalemia [Table 1] and normocalcemia. ABG showed metabolic alkalosis. Further workup in the context of metabolic alkalosis and hypokalemia revealed high aldosterone to renin ratio with high serum aldosterone and low renin activity [Table 1]. A suspicion of primary hyperaldosteronism led to CT abdomen which revealed bilateral renal MNC and mass lesion involving left adrenal gland [Figure 3a and b]. These findings confirmed primary hyperaldosteronism due to adrenal adenoma with metabolic alkalosis and MNC. In the further course, the patient underwent adrenalectomy and her symptoms and hypokalemia resolved and is currently under regular follow-up.

- (a) CT scan of the abdomen showing bilateral medullary nephrocalcinosis (MNC) in a patient with adrenal adenoma and (b) Left adrenal adenoma in a patient with nephrocalcinosis
Discussion
Sjogren's syndrome is an autoimmune disease with glandular andextra-glandular manifestations. Tubulointerstitial nephritis is the leading renal involvement linked with pSS.[3] It might manifest as dRTA that may cause hypokalemia and patient can present with recurrent but reversible episodes of myopathic weakness. dRTA is characterized by the inability of the distal nephron to acidify the urine to a pH of <5.3 under conditions of spontaneous or induced acidosis. RTA continues to be reported in 4.3–9% of pSS patients.[4] NC with RTA and hypokalemic paralysis as a presenting feature of pSS is described in only a few cases reports in the literature. dRTA happens to be the most frequent possible cause of NC as a consequence of hypercalciuria without hypercalcemia. The reported prevalence of NC in patients with dRTA usually ranges from 60 to 80%. dRTA most typically is associated with Sjogren's syndrome occurs in as many as 33% of patients.[5]
pSS may present itself as dRTA-associated NC by autoantibodies directed against the A-type intercalated cells within the collecting tubules causing a defect in hydrogen-ion secretion.[5] The mechanism of hypokalemia in Sjogren's syndrome can be attributed to dRTA triggered by chronic interstitial nephritis which ends up in decreased tubular sodium delivery, defective H-K ATPase, secondary hyperaldosteronism, and bicarbonaturia.[6] Our patients described incase 1 had repeated episodes of hypokalemia and metabolic acidosis in the past, which responded symptomatically to potassium supplementation alone. Thus, they were labeled as a case of hypokalemic periodic paralysis but detailed workup regarding the etiopathogenesis of their problem was missed. Although SS would likely have a varying clinical presentation, presentation of a person with renal symptoms in the form of hypokalemia and NC as the first symptom might create confusion to reach the diagnosis. This case highlights the importance of a high index of suspicion for the possibility of pSS, especially in patients who present with NC, hypokalemia, and metabolic acidosis. It should, thus, be a routine procedure to evaluate distal tubular function in pSS patients who has NC on presentation.
Primary hyperparathyroidism (PHPT) and parathyroid adenoma as a cause of PHPT are rare in a young person, PHPT has an incidence of 2–5/100,000 children.[7] A high index of suspicion is needed to identify it as a cause of MNC and kidney injury in a young individual.[8] Renal manifestations of PHPT include hypercalciuria, nephrolithiasis, NC, chronic renal insufficiency, and renal tubular dysfunction in the form of type 1 or 2 RTA.[9] Hypercalciuria is implicated in the complex pathophysiology of NC in PHPT. Our patient described in case 2 had a history of renal stones since childhood, and bilateral NC at the time of presentation. As the symptoms started at a young age primary dRTA and other tubulopathies, that is, familial hypocalcemic hypercalciuria (FHH) was ruled out by the presence of HH [Table 1].[10] Secondary HPTH was ruled out because of the absence of hyperphosphatemia and hypocalcemia. NC progression is essentially reversible following successful surgery for the cause[9] which highlights the significance of early diagnosis to stop disease progression and further complications. Our case highlights the NC as a presentation of parathyroid adenoma at a young age and should be included in the workup for early diagnosis and treatment.
MNC linked to hyperaldosteronism is rare with just a handful of cases described in the literature.[11] Hyperaldosteronism is known to facilitate calcium excretion in urine. Calcium excretion is linked with Na excretion in urine; for almost every 100 mEq/dL increment in Na excretion,[3] there is a 40-mg/dL increase in Ca excretion.[3] In hyperaldosteronism, there exists reduced reabsorption of Na from the aldosterone insensitive tubular sites, causing subsequent hypercalciuria. Also, chronic hypokalemia observed in cases of hyperaldosteronism can itself cause a tubulointerstitial injury. Metabolic alkalosis linked with hypokalemic states cuts down calcium phosphate or oxalate solubility in alkaline urine, predisposing to calcinosis.[11]
Case 3 highlights aldosterone secreting adrenal adenoma with associated MNC. In our case, metabolic alkalosis found on ABG first drew our attention toward the possibility of causes leading to hyperaldosteronism. Thus, chronic hyperaldosteronism secondary to any cause, not merely primary hyperaldosteronism is essential to be taken as a potential risk ingredient in NC and should be included as one of the differentials of NC.
Conclusion
This case series of three patients who had NC in common but had three different diagnoses. Hence, MNC can be a part of varied spectra of diagnosis, and extensive workup is often needed for reaching the diagnosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Nephrocalcinosis. 2019. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; Available from: https://www.ncbi.nlm.nih.gov/books/NBK53720
- [Google Scholar]
- The importance of the thick ascending limb of Henle'sloop in renal physiology and pathophysiology. Int J Nephrol Renovasc Dis. 2018;11:81-92.
- [Google Scholar]
- Renal presentation of primary Sjogren's syndrome: Hypokalemic paralysis with Type 1 distal renal tubular acidosis. Nephrol Dialysis Transplant. 2018;33:i51.
- [Google Scholar]
- Hypokalemic paralysis due to primary Sjogren syndrome. Indian J Endocrinol Metab. 2018;22:287-9.
- [Google Scholar]
- Primary hyperparathyroidism: A rare endocrinopathy in children. Two case reports. Endokrynol Pol. 2011;62:346-50.
- [Google Scholar]
- Atypical parathyroid adenoma in a young individual presented with nephrocalcinosis (Abstract) Endocr Abstr. 2018;56:260.
- [Google Scholar]
- Renal manifestations of primary hyperparathyroidism. Indian J Endocr Metab. 2012;16:258-62.
- [Google Scholar]
- An Association of chronic hyperaldosteronism with medullary nephrocalcinosis. Pol J Radiol. 2015;80:417-24.
- [Google Scholar]







