Translate this page into:
Nephrotic Syndrome with Central Retinal Artery Occlusion: A Unique Presentation
Address for correspondence: Dr. Anika Agrawal, Department of Pediatrics, Maulana Azad Medical College, New Delhi, India. E-mail: dranikaagrawal@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Childhood nephrotic syndrome is associated with significant morbidity because of recurrent relapses, infections, and episodes of thromboembolism. Thromboembolism in nephrotic syndrome may involve any major blood vessel. Timely recognition of symptoms and early initiation of anticoagulation therapy are important to avoid end-organ damage. We present here a case of a child with steroid-resistant nephrotic syndrome (SRNS) with bilateral central retinal artery occlusion (CRAO), whose vision improved with anticoagulation therapy.
Keywords
Anticoagulation
blindness
nephrotic syndrome
thromboembolism
Introduction
The incidence of nephrotic syndrome is 1.52 per 100,000 living children per year.[1] Glucocorticoids are the standard initial therapy for nephrotic syndrome. After infections, thromboembolism is considered by many experts to be the most significant life-threatening complication of nephrotic syndrome.[23] The hypercoagulable state in nephrotic syndrome is multifactorial, resulting from loss of anticoagulant proteins in the urine, increased procoagulant proteins, thrombocytosis, and enhanced platelet activation and aggregation.[456] Other factors that may also contribute are low albumin, increased cholesterol, associated infections, iatrogenic volume depletion because of inappropriate and overuse of diuretics, and immobilization.
Venous thromboembolism is more common than arterial thromboembolism. Any major blood vessel, including cerebral, renal, and peripheral vessels, may be involved. However, there are no case reports of an involvement of retinal vessels in nephrotic syndrome. Here, we describe a case report of a 10-year-old child who presented with nephrotic syndrome and bilateral complete loss of vision because of thromboembolism of bilateral central retinal artery occlusion (CRAO).
Case Report
A 10-year-old male child presented with anasarca and decreased urine output for 1 month. There was no history of fever, abdominal pain, cough, burning micturition, palpitation, breathlessness, or diarrhea. There was also no history of recent sore throat, rash, or joint pain.
On examination, there was no pallor, icterus, or lymphadenopathy. His blood pressure (BP) was normal. Periorbital edema, abdominal distention, and pitting pedal edema were present. The abdomen was soft, distended, and nontender. The chest and cardiovascular system examination were normal.
Investigations showed a hemoglobin of 10.6 gm/dl, hematocrit of 34%, total leucocyte count of 9800 cells/cu mm, differential count of P60L40, and a platelet count of 2.14 lac/cu mm. Serum albumin of 1.13 g/dl, serum cholesterol of 598 mg/dl, and protein dipstick of 4+. Urine examination showed significant proteinuria and no hematuria or pyuria. Spot urine protein creatinine ratio was 2.6. Hepatitis B (HBV) and hepatitis C (HCV) serology were negative. The antinuclear antibodies were negative. The complement component 3 (C3) levels were normal.
The child was diagnosed with the first episode of nephrotic syndrome and started on furosemide at 2 mg/kg/day for 5 days along with oral prednisolone at 2 mg/kg/day. One week after the initial presentation, the child complained of decreased vision in both eyes. The edema had decreased but not subsided completely. The BP was normal. Ophthalmological examination showed an absence of perception of light in both the eyes and normal pupillary reaction and intraocular pressures (IOPs). Fundus examination showed central pallid retina and cherry-red spots with dot-blot hemorrhages bilaterally, suggestive of CRAO [Figure 1a and b].

- Fundus of the right eye (a) and left eye (b) showing central pallid retina, cherry-red spots with dot-blot hemorrhages suggestive of bilateral central retinal artery occlusion
Urine protein by dipstick was 3+, serum albumin 2.2 g/dl, and serum cholesterol 559 mg/dl. Prothrombin time (PT), international normalized ratio (INR), and activated partial thromboplastic time (aPTT) were 10.9, 1.05, and 22.2 seconds, respectively. D-dimer was more than 5000 ngm/ml and serum fibrinogen was 287 mg/dl. The ultrasound showed normal-sized kidneys with normal echogenicity. The ultrasound Doppler showed normal renal flow. The magnetic resonance imaging (MRI) and angiography were essentially normal.
Prednisolone was continued in daily doses of 2 mg/kg/day and the child was started on low molecular weight heparin (1 mg/kg/dose 12 hourly subcutaneously) for 1 week followed by oral warfarin. The warfarin was initially started at a dose of 0.1 mg/kg/day and titrated to keep the target INR to 2–2.5.
Despite four weeks of steroids, proteinuria was persisting but the vision had improved to finger counting at 1 meter. The child was started on cyclosporine at 3 mg/kg/day and enalapril at 0.1 mg/kg/day. Warfarin and prednisolone were continued. Renal biopsy was deferred as the patient was on warfarin.
At 2 months since initiation of treatment, the child was in remission and vision was 3/60, 6/60 in left and right eye, respectively. D dimer was 460 ngm/ml. At 4 months since initiation of treatment, warfarin was stopped and a renal biopsy was done, which showed a mild increase in mesangial matrix and cellularity. Blood vessels showed medial sclerosis. There was no evidence of endocapillary proliferation or crescent formation. The immunofluorescence staining was negative for immunoglobulin G (IgG), IgM, IgA, C3, and C1q. The findings were suggestive of minimal change disease [Figure 2]. At the last follow-up at 6 months since the onset of disease, the child continued to be in remission. Visual acuity (VA) was 6/60 in both eyes with no field defects. The child was on prednisolone, cyclosporine, and enalapril. The plan was to taper and stop prednisolone and continue the child on cyclosporine and enalapril.
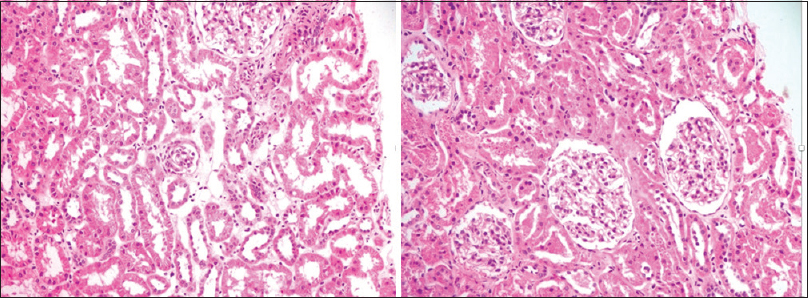
- Renal biopsy showing tubules and glomerular changes suggestive of minimal change disease (hematoxylin-eosin stain, 20X)
Discussion
Most children have steroid-sensitive nephrotic syndrome (SSNS), with about 20% of children having steroid-resistant nephrotic syndrome (SRNS).[7] In this 20% of the children, the treatment of choice is calcineurin inhibitors (tacrolimus/cyclosporine) and mycophenolate mofetil.[8]
As early as 1840, renal vein thrombosis was the first thromboembolism recognized to be associated with nephrotic syndrome. Since that time, it has been recognized that nephrotic syndrome-associated thromboembolism may be seen in essentially any major blood vessel.[9] In a study by Suri et al. on 34 patients of nephrotic syndrome with thromboembolic episodes, cerebral venous thrombosis was the most common complication seen in 11 (31.4%) children, followed by pulmonary thromboembolism and deep venous thrombosis (DVT) in 9 (25.7%) and 6 (16.6%) children, respectively.[5] Sinha et al. first reported thrombosis of the ophthalmic vessels in a 3-year-old child with nephrotic syndrome.[10]
The incidence of thromboembolism in nephrotic syndrome is only 3% in children compared with an incidence of 25% in adults.[3] In a systemic review by Tahouri et al., the pooled prevalence of thromboembolic events in children with nephrotic syndrome was estimated to be 6%.[4] Infants and children aged >12 years are at a much greater risk. Membranous changes in histopathology, steroid resistance, and steroid dependence are associated with an increased risk of thromboembolism.[5] Thromboembolic events most commonly occur within 3 months after the initial diagnosis.[4611]
Once thromboembolism has developed, clinical management includes early heparin therapy, followed by oral anticoagulants.[512] The total recommended duration of anticoagulation for first venous thromboembolism is at least 3–6 months and until nephrotic syndrome is in remission. There are no guidelines recommending prophylactic anticoagulation to prevent thromboembolism in nephrotic syndrome. As the majority of patients do not develop thromboembolism, a significant number of patients would be exposed to potential adverse effects like bleeding unnecessarily. Therefore, the risk–benefit ratio should be analyzed for each patient individually before considering thromboembolism preventive therapy.[4111314] Statins may have a role in the prevention of thromboembolism; however, more studies are needed.[15]
Conclusion
Thromboembolism in nephrotic syndrome can essentially involve any major blood vessel. A high index of suspicion is required as the clinical features may be subtle. Inducing remission and starting timely anticoagulation therapy can decrease the risk of end-organ damage and associated mortality.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Nephrotic syndrome in The Netherlands: A population-based cohort study and a review of the literature. Pediatr Nephrol. 2011;26:1241-6.
- [Google Scholar]
- Nephrotic Syndrome Nelson Textbook of Pediatrics. (Edition 20). Philadelphia, PA: Elsevier; 2016. p. :2521-3.
- [Google Scholar]
- Epidemiology and pathophysiology of nephrotic syndrome–associated thromboembolic disease. Clin J Am Soc Nephrol. 2012;7:513-20.
- [Google Scholar]
- Thromboembolism due to nephrotic syndrome among children; A systematic review and meta-analysis. J Renal Endocrinol. 2018;4:17.
- [Google Scholar]
- Thromboembolic complications in childhood nephrotic syndrome: A clinical profile. Clin Exp Nephrol. 2014;18:803-13.
- [Google Scholar]
- Hemostatic complications in renal disorder of the young. Pediatr Nephrol. 1996;10:88-99.
- [Google Scholar]
- Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr Nephrol. 2001;16:1040-4.
- [Google Scholar]
- Treatment of steroid-resistant nephrotic syndrome in children– New guidelines from KDIGO. Pediatr Nephrol. 2013;28:409-14.
- [Google Scholar]
- Venous Thromboses at Unusual Sites: Consultative Hemostasis and Thrombosis (2nd ed). Philadelphia: Saunders Elsevier; 2007. p. :263-4.
- Bilateral combined central retinal artery and vein occlusion in a 3-year-old child with nephrotic syndrome. Indian J Ophthalmol. 2018;66:1498-501.
- [Google Scholar]
- Epidemiology and risk factors for thromboembolic complications of childhood nephrotic syndrome: A Midwest Pediatric Nephrology Consortium (MWPNC) study. J Pediatr. 2009;155:105-10.
- [Google Scholar]
- Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed.: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e737S-801S.
- [Google Scholar]
- High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: Results from a large retrospective cohort study. Circulation. 2008;117:224-30.
- [Google Scholar]
- Prophylactic anticoagulation in nephrotic syndrome: A clinical conundrum. J Am Soc Nephrol. 2007;18:2221-5.
- [Google Scholar]
- Statin use in patients with nephrotic syndrome is associated with a lower risk of venous thromboembolism. Thromb Res. 2011;127:395-9.
- [Google Scholar]







