Translate this page into:
Nutrition Compass: Guiding Patients with Chronic Kidney Disease Across Ages
Corresponding author: Sukanya Govindan, Department of Pediatric Nephrology, Mehta Multispeciality Hospitals India Private Limited, Chennai, Tamil Nadu, India. E-mail: sukanyagovindan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Govindan S, Iyengar A, Mohanasundaram S, Priyamvada PS. Nutrition Compass: Guiding Patients with Chronic Kidney Disease Across Ages. Indian J Nephrol. 2025;35:187-97. doi: 10.25259/IJN_222_2024
Abstract
Malnutrition, encompassing both undernutrition and overnutrition, is prevalent among patients with chronic kidney disease (CKD). It is influenced by a myriad of factors, including dietary restrictions, metabolic irregularities, inflammation, and comorbidities. It leads to increased morbidity, mortality, and diminished quality of life. In children, malnutrition hinders growth and development, particularly during infancy and early childhood. This article provides a comprehensive overview of current terminologies delineating undernutrition and overnutrition in CKD, and discusses age-specific nutritional assessment tools. It delves into macro- and micronutrient prescriptions tailored for both adults and children with CKD, emphasizing special considerations such as low and very low protein diets. A focus on the nutrient content of Indian foods is also provided, alongside available nutritional supplements, with insights into enteral feeding and the fortification of feeds in young children. Distinctive nuances in nutritional therapy between adults and children are elucidated throughout the article, drawing upon established guidelines, including Kidney Disease Outcomes and Quality Initiative (KDOQI) 2009 and 2020 and KDIGO CKD 2024 guidelines as well as Pediatric Renal Nutrition Task Force clinical practice recommendations, to inform dietary management strategies for individuals across the lifespan living with CKD.
Keywords
Chronic kidney disease
Dietary management
Indian diet
Nutritional assessment
Pediatric CKD
Introduction
Undernutrition in chronic kidney disease (CKD) involves an interplay of a multitude of factors like decreased appetite, reduced food intake, dietary restrictions to account for reduced kidney function, inadequate dialysis, dialysis losses, inflammation, hormonal alterations, and metabolic abnormalities. Due to persistent inflammatory state, patients with CKD have higher resting energy expenditure leading to protein breakdown and decreased anabolism.1 Obesity is increasingly recognized, both as a risk factor for CKD and contributing to poor clinical outcomes of CKD.2
Malnutrition is associated with increased mortality and morbidity in patients with CKD.1,3 Patients with CKD and malnutrition experience more infections compared to patients with no malnutrition (62% versus 29%) and have prolonged hospitalization (14 days versus 10 days).4 This is caused by immunodeficiency, which increases the risk of infections and impairs wound healing. The overall mortality due to CKD is increased in patients with poor nutritional status. Poor energy intake of <25 kcal/kg and malnutrition inflammation score (MIS) of >5 are independent predictors of mortality in patients with CKD.5
There are important differences between children and adults with CKD. Children have the onset of CKD prior to achieving full growth potential, and stunted growth (height <2SD) is a major complication affecting almost 35–60% of children.6,7 Both linear growth and weight in children are strongly influenced by nutrition; this effect is more pronounced in infants and toddlers. Young children have unique dietary needs like the need for a predominantly liquid-based feeds and augmented feeding methods like the enteral feeding in infants to ensure optimal nutrition.8 Children with tubular and interstitial disorders often have salt wasting and polyuria requiring fluid replacement, salt, and electrolyte supplementation. Similar to adults with CKD, children with short stature, underweight, and obesity have higher all-cause mortality compared to children with normal height and normal weight patients.9
Common definitions used in nutrition in CKD
Malnutrition: It is a state in which deficiency of nutrients such as energy, protein, vitamins, and minerals causes measurable adverse effects on body composition, function, or clinical outcome. The term malnutrition includes both undernutrition and overnutrition.10
Undernutrition: The American Society for Parenteral and Enteral Nutrition (ASPEN) defines undernutrition as the presence of two or more of the following six evidence-based criteria for nutritional assessment, viz. insufficient energy intake, weight loss, loss of muscle mass, loss of subcutaneous fat, fluid accumulation that can sometimes mask weight loss and diminished “functional status” as measured by hand grip strength.11
Protein energy wasting
The International Society of Renal Nutrition and Metabolism (ISRNM) defines protein energy wasting (PEW) in adults as the state of decreased body stores of protein and energy fuels (i.e., body protein and fat masses). It is diagnosed using four main categories: biochemical criteria; low body weight, reduced total body fat, or weight loss; a decrease in muscle mass; and low protein or energy intakes.12 There is no well-defined or accepted criteria for PEW in children. Table 1 shows the criteria for PEW in adults with CKD.
| Parameter | Criteria |
|---|---|
| Serum chemistry |
Serum albumin <3.8 g/dl Serum prealbumin (transthyretin) <30 mg/dl (for maintenance dialysis patients only; levels may vary according to GFR level for patients with CKD stages 2–5)a Serum cholesterol 100 mg/dla |
| Body mass |
BMI <23 kg/m2b Unintentional weight loss over time: 5% over three months or 10% over six months Total body fat percentage <10% |
| Muscle mass |
Muscle wasting: reduced muscle mass 5% over three months or 10% over six months Reduced mid-arm muscle circumference areac (reduction >10% in relation to 50th percentile of reference population) Creatinine appearanced |
| Dietary intake |
Unintentional low DPI <0.80 g kg/day for at least two monthse for dialysis patients or <0.6 g kg/day for patients with CKD stages 2–5 Unintentional low DEI <25 kcal kg/day for at least two monthse |
DPI: Dietary protein intake, DEI: Dietary energy intake aNot valid if low concentrations are due to abnormally great urinary or gastrointestinal protein losses, liver disease, or cholesterol-lowering medicines.bA lower BMI might be desirable for certain Asian populations; weight must be edema-free mass, for example, post-dialysis dry weight. See text for the discussion about the BMI of the healthy population. cMeasurement must be performed by a trained anthropometrist. dCreatinine appearance is influenced by both muscle mass and meat intake. eCan be assessed by dietary diaries and interviews or for protein intake by calculation of normalized protein equivalent of total nitrogen appearance (nPNA or nPCR) as determined by urea kinetic measurements. PEW: protein energy wasting, CKD: chronic kidney disease, BMI: body mass index.
Cachexia: This term is used to refer to severe forms of PEW.
Overweight and Obesity: Overweight is defined as a body mass index (BMI) of 25–29.9 kg/m2 and obesity as a BMI of ≥30 kg/m2.13
Metabolic syndrome: Metabolic syndrome is defined as the presence of overweight or obesity and at least two of four additional CV risk factors:
-
1.
Systolic and/or diastolic office blood pressure (BP) ≥90th centile for age, sex, and height or ≥130/80 mmHg, whichever is lower, or on antihypertensive medication
-
2.
Fasting triglycerides ≥100 mg/dL (1.1 mmol/L) if age <10 years or ≥130 mg/dL (1.5 mmol/L) if age ≥10 years
-
3.
Fasting high-density lipoprotein (HDL) <40 mg/dL (1.03 mmol/L)
-
4.
Fasting serum glucose ≥100 mg/dL (5.6 mmol/L) or known type 2 diabetes mellitus (T2DM)14
Definitions in children
The World Health Organization (WHO) defines stunting as height/length for age less than third centile or <2SD–3SD and severe stunting as height/length for age <3SD, underweight as weight for age less than third centile or <2SD–3SD, and severely underweight as <3 SD.15
Overweight and obesity: For children aged two to five years, PRNT guidelines define overweight as weight-for-height for age >2SD, using the WHO child growth standard chart and obesity as weight-for-height for age >3SD. For children aged more than five years, overweight is defined as BMI for age >1SD, equivalent to BMI >25 kg/m2 at 19 years. Obesity is defined as BMI for age >2SD, equivalent to BMI >30 kg/m2 at 19 years, using the WHO growth reference chart or a country-specific growth chart.14
The nutrition care process involves multiple components, such as periodic monitoring, assessment of various parameters, which leads to diagnosis, culminating in intervention [Figure 1].16
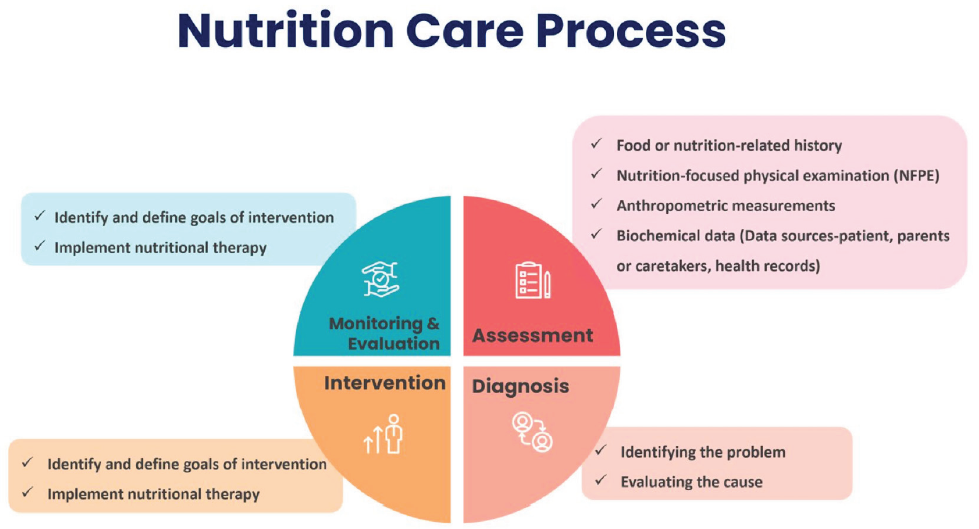
- Components of nutrition care process. Modified from reference 16.
Nutritional Assessment Tools
Dietary assessment
A comprehensive dietary assessment is required in patients with CKD. The assessment aims to gather information on total energy and protein intake along with macro- and micronutrient intake. Details about salt intake, salt substitutes, overall fluid intake, cooking methods, and eating patterns should be elicited, as this is particularly relevant in patients with CKD. It is also important to note details of medications like calcium supplements, potassium-binding resins, liquid medications, and protein supplements that a patient might be taking in addition to diet. As dietary assessment is self-reported, under- and overreporting is a concern.
Food record/diary: A three-day prospective food record is the preferred method to assess dietary intake. In dialysis patients, this should include both dialysis and non-dialysis days. In children, the three-day record should include weekdays and weekends to account for diet variations during school and nonschool days. The patient or caregiver should be advised to note down portion sizes of meals to improve the accuracy of the dietary assessment. Incorporating smart phone-based applications can facilitate real-time diet capture, portion size, and save time.17
Diet recall: A 24-hour dietary recall is an alternative method to assess dietary intake when a prospective food diary is not feasible. This method can also be utilized to include multiple 24-hour period diet recall.
Food frequency questionnaires: FFQs use a finite list of foods and beverages and estimate the frequency of consumption of each item. This method is most useful when applied at a community level and to get information on dietary patterns rather than get individual patient-specific dietary information.17,18
Anthropometry
Anthropometric measures are an important part of the nutritional assessment in children to evaluate growth and in adults to assess body composition. The recommended anthropometry measurements in adults and children with CKD are given in Supplementay Table 1.
Complementary Nutritional Assessment Tools
Composite scoring system in adults
The routine assessment of nutrition in adults with CKD 3–5D should be considered at least biannually to identify those at risk of PEW. The National Kidney Foundation Kidney Disease/Dialysis Outcomes and Quality Initiative (NKF KDOQI)18 quotes that there is limited evidence in the utility of one assessment tool over another for this purpose.
Subjective Global Assessment (SGA): This tool helps not only in the assessment of nutritional status assessment but also in predicting nutrition-related clinical outcomes, such as infection rates and mortality.19 SGA has several advantages in clinical and research settings, including its low cost, quick implementation, adaptability to diverse disciplines (e.g., nurses, dietitians, and physicians), and proven reproducibility, validity, and reliability in studies. The seven-point SGA uses five criteria from history (weight loss in kilograms over the last six months, dietary intake in the last two weeks, gastrointestinal symptoms, nutrition-related functional status, and disease state affecting nutritional status), and three physical examination criteria (muscle wasting, fat depletion, and nutrition-related edema) scored on a seven-point scale and further classifies patients into three categories (A = well nourished, B = moderately malnourished, and C = severely malnourished). Given its strengths, KDOQI18 recommends the use of seven-point SGA19 as a valid and reliable tool for assessing nutritional status in adults with CKD undergoing dialysis.
Malnutrition Inflammation score: Another composite scoring tool, MIS incorporates ten components from medical history (change in dialysis dry weight over three to six months, dietary intake, gastrointestinal symptoms, functional capacity, and comorbidities, including time on dialysis), physical examination (muscle wasting and loss of subcutaneous fat), BMI, and laboratory parameters (serum albumin and serum total iron binding capacity [TIBC]) in assessing malnutrition. The KDOQI guidelines suggest that MIS may be used in the assessment of nutritional status.18
There are multiple condensed forms of these tools used in clinical studies. So the adoption of a uniform assessment tool in a population, chosen randomly, which is representative of the entire country, would facilitate the incorporation of these tools in regular clinical practice.
Body composition assessment: The assessment of body composition can be affected by the volume status of the patient and also the time of assessment. Understanding these subtle differences helps in the apt utility of the technical devices in body composition assessment. Multifrequency bioimpedance (MF-BIA) can be used for body composition assessment in adult patients on maintenance hemodialysis (MHD) and should be ideally done after a minimum of 30 minutes after the end of the hemodialysis session, so as to facilitate the redistribution of body fluids. There is insufficient evidence for its use in patients on PD and CKD patients, not on dialysis and in pediatric CKD patients. Dual-energy X-ray absorptiometry (DXA) is considered the gold standard for body composition assessment. Despite being labor-intensive, invasive, and expensive as well as being influenced by volume status, this is a valid technique used for patients on HD and PD. DXA is reserved in pediatric CKD only for research purposes.17,18
Complementary tools in children
Subjective global nutritional assessment (SGNA)
Components: The SGNA incorporates a comprehensive blend of subjective and objective elements from both medical history and physical examination to evaluate the extent of undernutrition. This tool encompasses ten parameters, with seven items derived from medical history and three from physical examination, all aimed at screening for undernutrition.17,20,21 The Pediatric Renal Nutrition Task Force (PRNT) suggests incorporating the nutrition-focused physical examination (NFPE) component of SGNA as a complementary assessment tool for children with CKD.22,23 NFPE entails visually and manually inspecting potential areas of fat or muscle wasting to gauge nutritional status. Assessment for specific micronutrient deficiencies should be considered if abnormalities are detected during the physical examination [Figure 2].
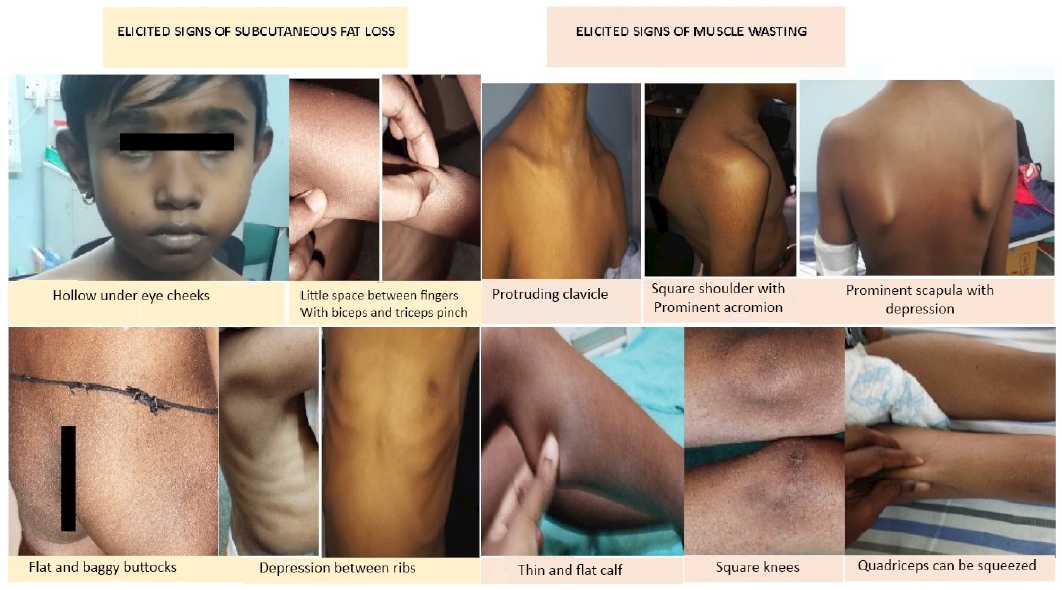
- Nutrition-focused examination signs for subcutaneous fat loss and muscle wasting in children with CKD. CKD: chronic kidney disease.
Biochemical parameters
Creatinine kinetics: Creatinine kinetics is used to measure muscle in adult patients with CKD. It is done using a 24-hour urine sample or calculated based on pre- and post-HD serum creatinine measurements in MHD patients who are anuric. It can be influenced by intake of meat or protein supplements containing creatine. In MHD patients, creatinine kinetics based on pre- and post-HD serum creatinine measurements is more reliable for anuric patients.18
Normalized Protein Catabolic Rate (nPCR): nPCR stands as a validated tool, specifically endorsed for adolescents and adults undergoing maintenance HD. A nPCR value exceeding one signifies sufficient protein intake and is a predictor of serum albumin and mortality in MHD patients.17,18 nPCR = 5.43 × estimated G/V1 = 0.17; Estimated G (mg/min) = [(C2 × V2) – (C1 × V1)]/T; where C1 = post-dialysis BUN(mg/dl); C2 = pre-dialysis BUN (mg/dl); V1 = post-dialysis total body water (dl) (0.58 × weight in kilograms); V2 = pre-dialysis total body water (dl) (0.58 × weight in kilograms); T = time from the end of dialysis to the beginning of the next dialysis (min).
Serum albumin serves as a pivotal nutritional marker in adults and children with CKD, albeit its interpretation necessitates the exclusion of non-nutritional causes of hypoalbuminemia such as fluid overload, protein loss, inflammation, and concurrent liver disease. Hypoalbuminemia is a predictor of mortality in patients undergoing dialysis.17,18
Serum prealbumin concentration shows association with nPCR, lean body, and fat tissue indices, and is associated with hospitalization and mortality in adult MHD patients.18 Figure 3 shows a comprehensive list of nutrition assessment tools based on age of the patient from infancy to adulthood.
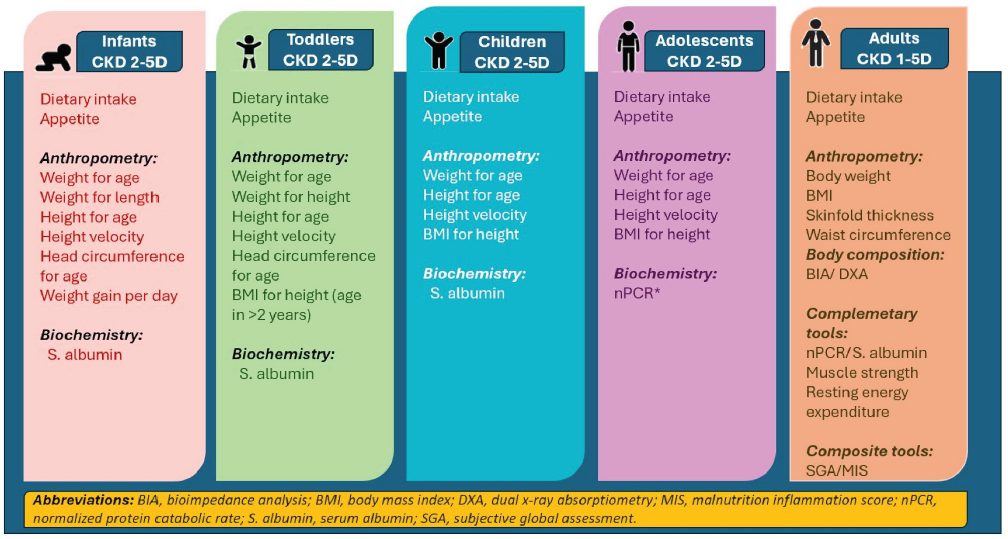
- Recommended tools for nutrition assessment in CKD across the spectrum of childhood and adulthood. Modified from reference 20. CKD: chronic kidney disease, *: maintenance hemodialysis.
Management
The body weight used for dietary calculations should be individualized based on clinicians’ judgment and related to patients’ health goals. If the body weight is <95% or more than 115% of the standard body weight, it is recommended to adjust it for nutritional recommendations using the formula 1.18
Adjusted BW = Ideal Body weight + [(Actual Body weight − ideal Body weight) × 0.25]. If the actual body weight cannot be determined due to fluid retention, the edema-free body weight can be used instead.18
Macro- and Micronutrient requirements in metabolically stable adult patients with CKD
The following recommendations apply to metabolically stable people [Supplementay Table 2]. In patients’ intercurrent medical issues, hospitalizations, and more, the requirements might vary depending on the nature of the intercurrent illness. For those with PEW, protein and energy intake should be increased till wasting subsides. A low protein diet (LPD) should not be started in a catabolic state.
Macro- and micronutrient prescription in children with CKD
There are many differences in prescribing diet for children with CKD when compared to adult CKD patients. A significant number of children with CKD have salt wasting, and hence need a normal to high salt diet with liberal fluid intake. Also, fats can be added to augment energy intake in most children unlike in adults, except in hyperlipidemia causing disorders like nephrotic syndrome. In adults with CKD, LPD has been shown to delay progression of CKD; however, protein restriction is not advised in children as they need positive nitrogen balance to support growth.24 Supplementay Table 3 summarizes macro- and micronutrient requirements as per the PRNT and KDOQI recommendations for children.25-27
The content of potassium and phosphorus in various food sources have been depicted in Figures 4 and 5.28 The potassium content of various food items found in the Indian diet is shown in Figure 6.
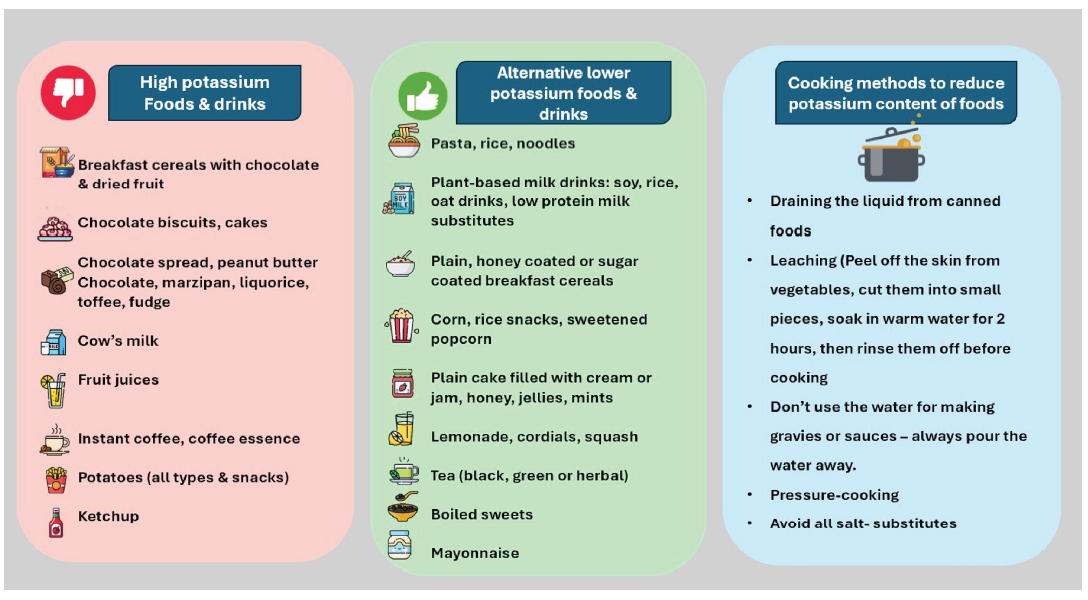
- Various sources of high potassium diet and alternative lower potassium foods.
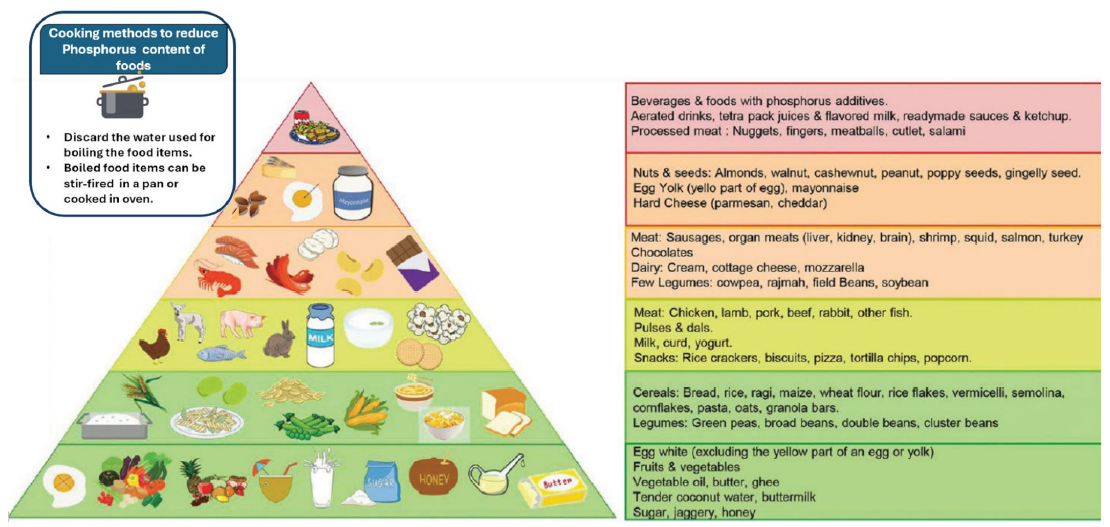
- Phosphorus pyramid. Adapted from reference 28.
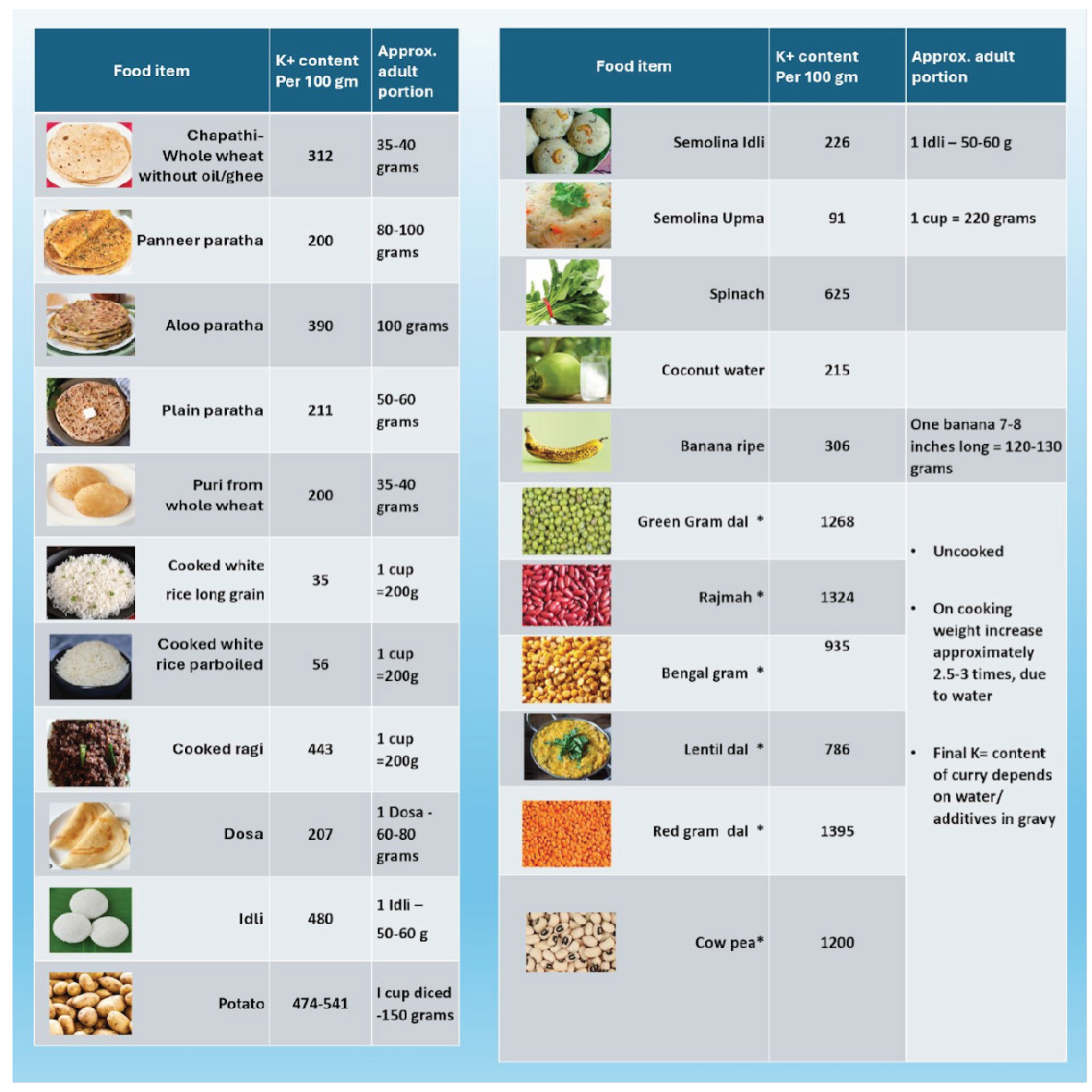
- Potassium content in various food sources from an Indian diet. *: Uncooked food item.
Protein restriction in adults with CKD—Rationale
Multiple observational studies have reported that a dietary protein intake (DPI) >1.2 g/kg body weight leads to a higher annual glomerular filtration rate (GFR) decline in CKD.29 The proposed benefits of LPD in CKD include reduction in intraglomerular pressures via afferent arteriolar constriction, reduced generation of urea and other nitrogenous waste products, reduced generation of indole and cresol derivatives by gut bacteria, lesser amount of acidosis, lesser phosphorus load, and reduced insulin resistance. LPD also contributes to reductions in proteinuria. Because of these, an LPD is considered beneficial in those with CKD or at risk of CKD and even with solitary kidneys. Based on metabolic studies, a protein requirement of 0.6 g/kg body weight, with at least 50% high biological value protein, is sufficient to meet the nutritional requirements of an adult. Diabetic patients might need a DPI of 0.6–0.8 g/kg body weight per day to facilitate optimal glycemic control and maintain nutritional status.30 LPD or its modifications should not be advised to children, adults with intercurrent medical illness and hypercatabolic conditions, baseline protein-energy wasting, eating disorders, or those with a limited life expectancy. Even though age is not a contraindication, close monitoring might be needed in people aged over 80 years. KDIGO 2024 guidelines31 recommend a DPI of 0.8 g/kg body weight in CKD stages G3–5, whereas KDOQI proposes slightly lower DPI18 [Supplementay Table 2].
Plant versus animal protein
Plant-based proteins are associated with better cardiovascular outcomes and reduced mortality in patients with heart diseases. The relatively lower protein content in plant-based diets protects against glomerular hyperfiltration in CKD and may lead to a lesser decline of GFR, delay dialysis, and improve patient survival.32 Vegetable proteins are more pH neutral—deficient in methionine and other sulfur-containing amino acids—and hence may reduce acid load and alleviate metabolic acidosis. In plant-based diets, phosphorus exists as complexes like phytates, which are not readily absorbed; hence, associated with a neutral P balance. Soy proteins are associated with a better lipid profile. The fiber in plant-based diets reduces gut dysgenesis and reduces the generation of gut-derived uremic toxins. Studies have shown that eating a fruit and vegetable diet is not associated with a higher risk of hyperkalemia; the alkalinizing effect of fruits and vegetables might protect against hyperkalemia.32
Very Low Protein Diet with Ketoanalogues
Ketoanalogues (KA) are calcium of essential amino acids; with the amino groups replaced by a keto group, they can enter metabolic pathways without generation of nitrogen. They can be converted to branched-chain amino acids by the urea cycle; the substantial amounts of leucine promote muscle protein synthesis. Even though different combinations are available, most of the KA contain keto salts of isoleucine, leucine, phenylalanine, and valine, one hydroxy acid of methionine, and tryptophan, threonine, histidine, and tyrosine. For CKD patients without diabetes, VLPD with KA can be considered. A VLPD diet (0.28–0.43g protein/kg body weight/day) with the remaining protein requirements (up to a total of 0.6g/kg body weight/day) supplemented by KA is associated with a lower risk of end-stage renal disease (ESRD) and death in non-diabetic CKD.18 An approximate dosing guideline is one tablet for every 5 kg of ideal body weight. Good quality data to suggest that the KA is not available in diabetic CKD.
Controversies on protein restriction in adults
Although many observational studies support LPD in adults with CKD, existing randomized controlled trials (RCT) do not support the beneficial effects of LPD in patients with kidney failure. Most of the beneficial impacts of RCT are limited to reduced deaths and delaying the onset of uremia and dialysis, whereas the effect on GFR preservation is not conclusive. Many RCTs, patient compliance, and adherence were major concerns; despite being prescribed <0.6g/kg body weight of proteins, patients often ended up taking around 0.68 g/kg body weight. Besides, all studies were focused on quantity of protein and not on quality.33,34 The KDIGO clinical practice guidelines 2024 strongly recommends protein restriction in metabolically stable, non-diabetic CKD 3–5 (level 1 A).31 However, in a Cochrane meta-analysis published the same year; the GRADE of evidence on protein restriction is not very strong; LPD is associated with moderate certainty in reducing death, low certainty in preventing CKD, and very low certainty in preventing GFR decline.30 KDOQI and KDIGO recommend different DPI in stable patients with CKD G 3–5; KDOQI advocates 0.55–0.6 g/kg body weight per day, whereas KDIGO 2024 recommends 0.8 g/kg body weight per day.31
Dietary considerations for CKD in the Indian context
The traditional Western diets contain around 1–1.4 g/kg body weight of proteins, the majority being of high biological value. However, Indians often overconsume cereals and underconsume protein sources like pulses/meat/fish and poultry.35 The Indian Council of Medical Research-National Institute of Nutrition (ICMR-NIN recommendations) 2020 suggest 0.83 mg/kg body weight per day of protein as the recommended daily allowance (RDA) in healthy adults with increments up to 1 g/kg body weight per day in vegetarians, to compensate for the lower quality of protein.35,36 The average Indian diet, barring the northeast region, contains <1 g/kg body weight of protein; across the rest of India, the net DPI ranges from 47 g to 58.5 g; and up to 50% of DPI is from cereals lacking lysine and threonine. The suboptimal pulse intake may contribute to deficiencies of methionine, cysteine and tryptophan. Even among Indian patients with CKD, who consume the RDA of protein, only 15–23% of it is of high biological value protein compared to a 50% requirement.37 Following a blanket protein restriction need not be necessary for all; the quality of protein also needs to be taken into account. Diet needs to be individualized, considering the sociocultural backgrounds and individual preferences, and should not be too restrictive. An overzealous dietary protein restriction may contribute to protein-energy wasting, perpetuating inflammation, and contributing to increased morbidity and mortality.37 Despite the significant regional variations in cuisine, the cereal-pulse-milk-based diet in India often leads to a higher intake of carbohydrate-derived calories with major deficits in protein.35,36,38,39 For healthy individuals, the ICMR-NIN recommends calories from cereals/millets to be limited to 45% of total calorie intake, 17% or more contributed by pulses/legumes/fish/meat and so on, 10% from milk, 5% from vegetables, 3% from fruits, 8% from nuts and seeds, and remaining 12% from fat and oils.38 However, Indians derive 51–65% of their daily calories from cereals and millet; more than 97% consume cereals over the recommended upper ranges. The contribution from pulses/legumes/meat/fish is only 11%; 36–44% of individuals from rural and urban areas eat a diet deficient in protein. High-income households also tend to eat less fruits and vegetables and depend more on cereals with a lesser intake of non-cereal sources of proteins.38 For a majority of people living in urban areas, processed foods contribute to >10% of total calorie intake. An average Indian consumes approximately 8 g of salt, despite being aware of the harmful effects of salt.39 The characteristics of the Indian diet provides unique opportunities as well as challenges in the field of medical nutrition therapy in CKD.
Diet also plays a main role in the management of mineral bone disease (MBD) in CKD, especially in reducing hyperphosphatemia and, consequently secondary hyperparathyroidism. Since higher calcium and phosphorus levels lead to higher calcium-phosphate cross-product formation, which leads to vascular calcification, and is also associated with higher cardiovascular morbidity, it is important to restrict dietary phosphorus within normal limits across all stages of CKD. Plant-based sources help in reducing dietary phosphate load, since the bioavailability of phosphorus from plant-based sources is relatively less compared to food that contains phosphate additives. Hence, a typical Indian vegetarian diet which is predominantly plant based does not warrant much modification similar to that of protein.35,39
Supplementary Nutrition in CKD
Patients with advanced stages of CKD, especially those on MHD and PD, might need nutritional supplements when dietary intake alone is insufficient to meet metabolic demands. Oral nutritional supplements (ONS) are prescribed initially for patients with CKD 3–5D to supplement dietary intake. Energy-dense, low electrolytes and kidney-specific preparations need to be used. ONS should not replace meals; rather, the patient should take ONS 2–3 times per day, preferably one hour after meals. In selected patients, intradialytic ONS/high protein meals can be used as a strategy to improve nutritional intake. Those who cannot meet the requirements orally are potential candidates for a trial of enteral feeding. Intradialytic parenteral nutrition (IDPN) is to be considered in patients on MHD with PEW who cannot meet the daily requirements through ONS/enteral feeding. IDPN is always supplementary and should be used as a bridge to improve nutritional status; an ideal candidate should be able to take approximately 20 kcal/kg body weight per day and a protein intake of 0.8 g/kg body weight per day. The presence of hypertriglyceridemia (TG >500 mg/dL) is a contraindication. If the daily nutrient intake is extremely poor, total parenteral nutrition (TPN) needs to be considered instead of IDPN. There are no guidelines on the optimal dosing of IDPN. The formulation can be single nutrient compounded bags or ready-to-use bags. As a general rule, electrolyte supplementation is not part of IDPN; hence, electrolyte-free solutions are preferred. The primary focus is on amino acids followed by energy; the total volume of infusate also needs to be considered to avoid volume overload. An amino acid delivery of at least 0.5 g/KBW should be targeted during each dialysis session, along with a maximum of 50–80 g of glucose and 30–50 g of lipids. There are no guidelines on the use of soy oil versus mixed oil lipid formulations. While introducing IDPN, the infused doses should be gradually escalated for over a week. Blood glucose, liver function tests, and triglyceride levels should be closely monitored while administering IDPN.18,40 There needs to be better quality studies on the impact of supplemental nutrition CKD. The enteral route is preferred over parenteral. Any intervention needs to be continued for at least three months to see any appreciable effects.
Augmented feeding in children
For infants with CKD 2–5D, the PRNT guidelines suggest that breast milk and infant formula is fortified when fluid restriction is desired or nutrient-dense food is required to meet growth requirements. Fortification can be done by concentrating on the existing formula or by adding formula to expressed breast milk or by adding glucose polymers and fat emulsions [Supplementay Table 4].41 Fortification should be done in a graded manner to avoid adverse effects like vomiting, diarrhea, and gastroesophageal reflux (GER) due to increased osmolality. Once correctable causes of poor oral intake like GER, acidosis, and inadequate dialysis are ruled out, the PRNT guidelines recommend that supplemental or exclusive enteral tube feeding should be commenced in children who are unable to meet their nutritional requirements orally. A gastrostomy tube is preferred over nasogastric tube feeding when long-term enteral feeding is needed.24,42
Management of Obesity in CKD Patients
Obesity is a major public health problem, and it affects a significant number of patients with CKD. As per the National Health and Nutrition Examination survey conducted between 2011 and 2014, 44% of patients with CKD in the United States have obesity.43 However, unlike management of undernutrition in CKD, there are huge gaps in our understanding and hence managing obesity in this population. Obesity hastens the progression of CKD and also increases morbidity due to CKD. The presence of obesity significantly impacts treatment delivery in terms of placement of temporary and permanent vascular access, increased risk of peritonitis, limited access to dialysis and transplantation, and more.2 The KDOQI guidelines suggest that overweight or obesity based on BMI be used as a predictor of lower mortality in CKD 5D MHD patients, although the mortality risk associated with overweight or obesity status (based on BMI) is not clear in patients with CKD 1–5.18
Treatment of obesity involves a multidimensional approach involving lifestyle modifications, anti-obesity medications, and bariatric surgery. Lifestyle interventions include modified diet, increased physical activity, and better sleep, and needs to be integrated into the overall management of the patient. Formulating a diet plan, given the CKD-specific restrictions on macro- and micronutrients, is challenging and needs access to renal dietitians and frequent monitoring of biochemical parameters. The recent KDOQI 2020 guidelines advice energy intake of 25–35 kcal/kg based on age, sex, activity level, and body weight goals. Overweight and obese patients should be prescribed the lower end of the recommended energy intake of 25 kcal/kg. Dietary interventions like Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean diet can be prescribed to adolescent and adult CKD patients with obesity along with moderate physical activity.14,18 Obesity and metabolic syndrome affects 15–30% of children with CKD. The management includes a comprehensive nutrition care plan involving physical activity, behavioral modification, and dietary adjustments. Unlike in adults, anti-obesity medications are not advised in children. It is recommended to prescribe a diet plan that has individualized energy intake adjusted for age, CKD stage, and dialysis to achieve weight loss in children without compromising overall nutrition.14
The nutritional management of patients with CKD is a vital component of their overall care, aimed at preserving kidney function, managing symptoms, preventing complications, and improving quality of life. A key component of nutritional therapy is using comprehensive age-appropriate nutritional assessment tools to identify deficits in diet and body composition. Dietary management of CKD patients should be customized to each patient based on dietary and nutritional assessment findings, biochemical parameters, and symptom complex. General principles in adults include providing adequate calories, vigilant protein restriction when needed, managing fluid intake, restricting sodium intake, and prescribing a diet low in phosphate and potassium when required. Children have unique dietary needs and need energy-dense food with normal or increased protein and maintain positive calcium balance to allow for normal growth. Fluid and electrolyte requirements are higher in the majority of children with CKD due to tubular pathology and dysplastic kidneys. Enteral feeding and fortification of feeds might be needed in infants and toddlers to address risk of growth failure due to inadequate nutrition. By adhering to these tailored dietary strategies, clinicians can optimize the nutritional status of CKD patients across the age spectrum, fostering better outcomes and improved overall well-being.
Conflicts of interest
There are no conflicts of interest.
References
- Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int [Internet].. 2013;84(6):1096-107. Available from: http://dx.doi.org/10.1038/ki.2013.147
- [CrossRef] [Google Scholar]
- Management of obesity in adults with CKD. J Am Soc Nephrol [Internet].. 2021;32(4):777-90. Available from: http://dx.doi.org/10.1681/ASN.2020101472
- [CrossRef] [Google Scholar]
- Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis [Internet].. 2001;38(6):1343-50. Available from: http://dx.doi.org/10.1053/ajkd.2001.29250
- [CrossRef] [Google Scholar]
- Malnutrition accelerates the occurrence of infectious complications in patients with chronic kidney disease: A cross-sectional survey of 682 patients with chronic kidney disease. Nutr Clin Pract [Internet].. 2023;38(5):1167-74. Available from: http://dx.doi.org/10.1002/ncp.11040
- [Google Scholar]
- Nutritional status predicts 10-year mortality in patients with end-stage renal disease on hemodialysis. Nutrients [Internet].. 2017;9(4):399. Available from: http://dx.doi.org/10.3390/nu9040399
- [CrossRef] [Google Scholar]
- Stature in children with chronic kidney disease: analysis of NAPRTCS database. Pediatr Nephrol [Internet].. 2006;21(6):793-9. Available from: http://dx.doi.org/10.1007/s00467-006-0040-7
- [CrossRef] [Google Scholar]
- Severe bone disease and low bone mineral density after juvenile renal failure. Kidney Int [Internet].. 2003;63(1):266-75. Available from: http://dx.doi.org/10.1046/j.1523-1755.2003.00727.x
- [CrossRef] [Google Scholar]
- Long-term outcome of infants with severe chronic kidney disease. Clin J Am Soc Nephrol [Internet].. 2010;5(1):10-7. Available from: http://dx.doi.org/10.2215/cjn.05600809
- [CrossRef] [Google Scholar]
- Associations of longitudinal height and weight with clinical outcomes in pediatric kidney replacement therapy: results from the ESPN/ERA Registry. Pediatr Nephrol [Internet].. 2023;38(10):3435-43. Available from: http://dx.doi.org/10.1007/s00467-023-05973-3
- [CrossRef] [Google Scholar]
- Overview | Nutrition support in adults | Quality standards | NICE. [cited 2024 Jul 14]; Available from: https://www.nice.org.uk/guidance/qs24
- Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) JPEN J Parenter Enteral Nutr [Internet]. 2012;36(3):275-83. Available from: http://dx.doi.org/10.1177/0148607112440285
- [Google Scholar]
- A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int [Internet].. 2008;73(4):391-8. Available from: http://dx.doi.org/10.1038/sj.ki.5002585
- [Google Scholar]
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--the evidence report. National institutes of health. Obes Res.. 1998;6(Suppl 2):51S-209S.
- [PubMed] [Google Scholar]
- Assessment and management of obesity and metabolic syndrome in children with CKD stages 2-5 on dialysis and after kidney transplantation-clinical practice recommendations from the Pediatric Renal Nutrition Taskforce. Pediatr Nephrol [Internet].. 2022;37(1):1-20. Available from: http://dx.doi.org/10.1007/s00467-021-05148-y
- [Google Scholar]
- Malnutrition database [Internet]. Micronutrients. [cited 2024 Jul 14]. Available from: https://platform.who.int/nutrition/malnutrition-database
- Nutrition care process and model update: Toward realizing people-centered care and outcomes management. J Acad Nutr Diet [Internet].. 2017;117(12):2003-14. Available from: http://dx.doi.org/10.1016/j.jand.2017.07.015
- [CrossRef] [Google Scholar]
- Assessment of nutritional status in children with kidney diseases-clinical practice recommendations from the Pediatric Renal Nutrition Taskforce. Pediatr Nephrol [Internet].. 2021;36(4):995-1010. Available from: http://dx.doi.org/10.1007/s00467-020-04852-5
- [Google Scholar]
- KDOQI Clinical Practice Guideline for nutrition in CKD: 2020 update. Am J Kidney Dis [Internet].. 2020;76(3 Suppl 1):S1-107. Available from: http://dx.doi.org/10.1053/j.ajkd.2020.05.006
- [CrossRef] [Google Scholar]
- A modified quantitative subjective global assessment of nutrition for dialysis patients. Nephrol Dial Transplant [Internet].. 1999;14(7):1732-8. Available from: http://dx.doi.org/10.1093/ndt/14.7.1732
- [CrossRef] [Google Scholar]
- Nutrition management for chronic kidney disease: Differences and special needs for children and adults. Semin Nephrol [Internet].. 2023;43(4):151441. Available from: http://dx.doi.org/10.1016/j.semnephrol.2023.151441
- [CrossRef] [Google Scholar]
- Validation of subjective global (nutritional) assessment (SGNA) in children with CKD. J Ren Nutr [Internet].. 2011;21(2):207. Available from: http://dx.doi.org/10.1053/j.jrn.2011.01.017
- [CrossRef] [Google Scholar]
- Nutrition-focused physical examination for detecting protein energy wasting in children with chronic kidney disease. Indian J Nephrol [Internet].. 2023;33(4):264-9. Available from: http://dx.doi.org/10.4103/ijn.ijn_145_22
- [CrossRef] [Google Scholar]
- Subjective global nutritional assessment [SGNA] in children on chronic dialysis- A prospective observational study. Indian J Nephrol [Internet].. 2022;32(4):334-41. Available from: http://dx.doi.org/10.4103/ijn.ijn_340_21
- [CrossRef] [Google Scholar]
- Energy and protein requirements for children with CKD stages 2-5 and on dialysis-clinical practice recommendations from the Pediatric Renal Nutrition Taskforce. Pediatr Nephrol [Internet].. 2020;35(3):519-31. Available from: http://dx.doi.org/10.1007/s00467-019-04426-0
- [Google Scholar]
- The dietary management of calcium and phosphate in children with CKD stages 2-5 and on dialysis-clinical practice recommendation from the Pediatric Renal Nutrition Taskforce. Pediatr Nephrol [Internet].. 2020;35(3):501-18. Available from: http://dx.doi.org/10.1007/s00467-019-04370-z
- [Google Scholar]
- The dietary management of potassium in children with CKD stages 2-5 and on dialysis-clinical practice recommendations from the Pediatric Renal Nutrition Taskforce. Pediatr Nephrol [Internet].. 2021;36(6):1331-46. Available from: http://dx.doi.org/10.1007/s00467-021-04923-1
- [Google Scholar]
- KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis [Internet].. 2009;53(3 Suppl 2):S11-104. Available from: http://dx.doi.org/10.1053/j.ajkd.2008.11.017
- [Google Scholar]
- Assessment of dietary phosphorus intake and implementation of parental phosphate education in pediatric chronic kidney disease. Indian J Nephrol [Internet].. 2023;33(3):188-94.
- [CrossRef] [Google Scholar]
- High-protein diet is bad for kidney health: unleashing the taboo. Nephrol Dial Transplant [Internet].. 2020;35(1):1-4. Available from: http://dx.doi.org/10.1093/ndt/gfz216
- [Google Scholar]
- Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst Rev [Internet].. 2020;10(11):CD001892. Available from: http://dx.doi.org/10.1002/14651858.CD001892.pub5
- [CrossRef] [Google Scholar]
- KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int.. 2024;105(4S):S117-314.
- [CrossRef] [PubMed] [Google Scholar]
- Plant-based diets in CKD. Clin J Am Soc Nephrol [Internet].. 2019;14(1):141-3. Available from: http://dx.doi.org/10.2215/CJN.08960718
- [CrossRef] [Google Scholar]
- Dietary protein restriction in CKD: the debate continues. Am J Kidney Dis [Internet].. 2009;53(2):189-91. Available from: http://dx.doi.org/10.1053/j.ajkd.2008.12.014
- [CrossRef] [Google Scholar]
- Protein restriction in adults with chronic kidney disease, with or without diabetes: Integrated Diabetes and Endocrine Academy (IDEA) consensus statement for Indian patients. Diabetes Metab Syndr [Internet].. 2023;17(5):102785. Available from: http://dx.doi.org/10.1016/j.dsx.2023.102785
- [CrossRef] [Google Scholar]
- Res.in. [cited 2024 Jul 15]. Available from: https://www.millets.res.in/pdf/what_india_eats.pdf
- A comparison of the Indian diet with the EAT-Lancet reference diet. BMC Public Health [Internet].. 2020;20(1):812. Available from: http://dx.doi.org/10.1186/s12889-020-08951-8
- [CrossRef] [Google Scholar]
- Nutritional status in stage V dialyzed patient versus CKD patient on conservative therapy across different economic status. Ren Fail [Internet].. 2014;36(3):384-9. Available from: http://dx.doi.org/10.3109/0886022X.2013.872570
- [CrossRef] [Google Scholar]
- Res.in. [cited 2024 Jul 15]. Available from: https://www.nin.res.in/RDA_Full_Report_2020.html
- Awareness, behavior, and determinants of dietary salt intake in adults: results from the National NCD Monitoring Survey, India. Sci Rep [Internet].. 2023;13(1):15890. Available from: http://dx.doi.org/10.1038/s41598-023-42694-x
- [CrossRef] [Google Scholar]
- Intradialytic parenteral nutrition for patients on hemodialysis: when, how and to whom? Clin Kidney J [Internet] 2023. ;16(1):5-18. Available from: http://dx.doi.org/10.1093/ckj/sfac171
- [Google Scholar]
- Practical guide A. Energy and protein requirements in children with CKD stages 2-5 and on dialysis [Internet]. Vitaflo-via.com. [cited 2024 Jul 15]. Available from: https://www.vitaflo-via.com/sites/default/files/2022-07/energy-and-protein-practical-guide_faw.pdf
- Delivery of a nutritional prescription by enteral tube feeding in children with chronic kidney disease stages 2-5 and on dialysis-clinical practice recommendations from the Pediatric Renal Nutrition Taskforce. Pediatr Nephrol [Internet].. 2021;36(1):187-204. Available from: http://dx.doi.org/10.1007/s00467-020-04623-2
- [Google Scholar]
- Trends in obesity among adults in the United States, 2005 to 2014. JAMA [Internet].. 2016;315(21):2284. Available from: http://dx.doi.org/10.1001/jama.2016.6458
- [CrossRef] [Google Scholar]







