Translate this page into:
Optimizing the Immunosuppression: Role of Early BK Polyomavirus Surveillance and Evolving a Cost-Effective Strategy
Corresponding author: Sandeep Mahajan, Department of Nephrology, All India Institute of Medical Sciences, New Delhi, Delhi, India. E-mail: mahajansn@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Subbiah AK, Mahajan S. Optimizing the Immunosuppression: Role of Early BK Polyomavirus Surveillance and Evolving a Cost-Effective Strategy. Indian J Nephrol. 2025;35:324-5. doi: 10.25259/IJN_528_2024
BK polyomavirus (BKPyV) are ubiquitous DNA viruses belonging to the family polyomaviridae. Primary infections typically occur in childhood and by the time of transplantation a significant proportion (75–90%) of adults are seropositive. Following primary infections, the virus remains latent, particularly in renal tubular cells and gets reactivated in an immunocompromised state. If left untreated, BKPyV infection can lead to BK virus nephropathy (BKVAN), resulting in progressive graft dysfunction, premature graft loss, and potentially causing ureteric strictures and an increased risk of urothelial malignancies. In the first year after transplantation, the prevalence rates are 30%–40% for viruria, 10%–20% for viremia, and 5%–10% for BKVAN.1 Though BK virus nephropathy was initially described in a kidney transplant recipient in 1971, there are no effective treatment or prophylaxis options or a clear-cut management strategy for BKPyV. Mainstay of treatment remains reduction in immunosuppression with its associated risks. Recent studies therefore, emphasize the importance of monitoring and early intervention strategies to manage BKPyV effectively, but this strategy is limited by cost and resource requirement.
In a recent issue of IJN, Sulaiman et al., in a single-center retrospective study, explore the utility of an early detection strategy for BKPyV infection using universal assessment of BKV viruria at second month post-transplant. In their protocol, they checked for viremia and subsequent monthly viruria. Test for viremia was done only if the initial viruria was significant (≥107 copies/mL),2 thus limiting the number of patients tested. Further, optimization of immunosuppression was done in patients with persistent viruria and viremia or biopsy-proven BKVAN. They observed significant BK viruria in 89 of the 529 patients studied and viremia in 56 and BKVAN in 14 patients. Subsequent decrease in immunosuppression was done in 58 of the 89 patients with viruria and all patients with viremia and BKVAN. This strategy led to clearance of viruria in three-fourths and viremia in two-thirds of the patients at 6 months, with comparable eGFR at 6 and 12 months as compared with BKV viruria negative patients. There was no graft loss due to BKVAN in this study. However, patients with viremia had higher hospitalizations due to other infections while no such trend was observed in viruric patients.
Chon et al., have shown that viruria precedes viremia by 7 weeks and high grade viruria predicts risk for viremia and nephropathy.3 This study also compared urine and plasma BKPyV levels and noted that the trend of both samples was similar. However, the cut-offs used in this study for high-grade viruria were ≥25 million copies/mL, unlike the conventional definition (≥107 copies/mL).
Though BK viruria precedes viremia and BKVAN by 4–12 weeks, it is not recommended as part of routine screening in clinical guidelines. This is due to the following drawbacks of using a urine sample-based screening strategy:
1. Fluctuations in urine viral loads, physiological variations in urine composition, and possibility of viral loads being outside the linear range of assays.
2. Need for re-confirmation by blood viremia measurements, prior to change in treatment adding on to the cost and repeated visits. A study evaluating the cost–benefit of such a strategy did not find urine testing to be cost-efficient in BKPyV detection and monitoring.4
3. Studies have not shown that isolated viruria increases the risk of BKVAN. Only a small percentage of patients with BKV viruria develop BKVAN. In the CERTAIN registry study, while around 25% patients had BKPyV viruria, only 3% developed BKVAN.5
4. There is no evidence supporting the reduction of immunosuppression for isolated viruria, which needs to be balanced with the risk of rejection specially in the first year after transplantation.
There have been no controlled trials to compare any of the screening strategies and its utility in graft survival. The recommendations for BKV screening [Table 1]1,6,7 are based on observational data and expert consensus.
| Guideline | Recommendations for BKV screening |
|---|---|
| KDIGO guideline (2009)6 | QNAT monthly for 6 months f/b 3 monthly till 1-year post-transplant |
| American Society of Transplantation (2019)7 | Monthly BKPyV-DNAemia screening till 9th month f/b 3 monthly till 2 years post-transplant |
| International Consensus guideline (2024)1 |
KDIGO: Kidney diseases improving global outcomes; QNAT: Quantitative nucleic acid testing, BKV: BK virus, f/b: followed by, BKPyV-DNAemia: BKPolyoma virus DNA levels
Though economic evaluation analysis has shown the financial benefit of routine BKPyV screening, they are yet to find a place in most of the resource limited settings due to lack of local cost-effectiveness data. Moreover, testing is not widely available.
A less-intensive screening strategy measuring viremia every 3 months in the first-year post-transplantation was evaluated in an observational study from our centre.8 In these 62 low/intermediate immunologically risk patients, sustained BK viremia was found only in nine patients (14.5%). Even though the immunosuppression was not altered based on BKV viremia, five out of nine cleared the virus while the remaining four patients had persistent viral load of <103 copies at the end of 1 year while none of them developed BKVAN.
This article by Sulaiman et al.2 suggesting the use of urine screening as a complementary approach to plasma screening and reducing immunosuppression in patients with persistent significant viruria may help in earlier diagnosis and more targeted testing, thus bringing down the overall cost of testing. The cost–benefit and graft outcomes beyond 1 year of such an approach is, however, still untested. A similar strategy looking at urine cytopathology for decoy cells, which has a high negative-predictive value (95%–100%), but low positive-predictive value (25%–30%) maybe useful as a more cost-effective screening technique in resource limited setting and should be evaluated in future prospective trials [Figure 1].
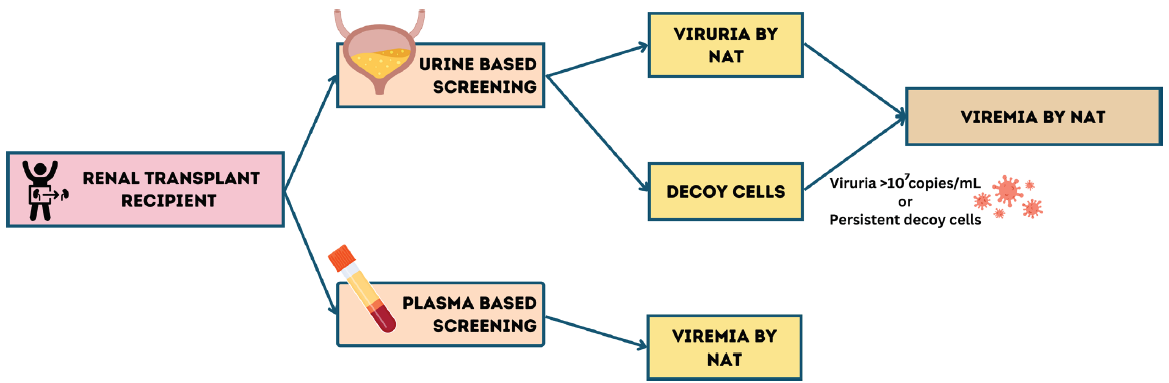
- Screening strategy for BK virus in post-transplant patients. NAT: Nucleic acid testing.
Conflicts of interest
There are no conflicts of interest.
References
- The second international consensus guidelines on the management of BK polyomavirus in kidney transplantation. Transplantation. 2024;108:1834-1866.
- [CrossRef] [PubMed] [Google Scholar]
- Early detection strategy of BK polyoma virus infection in kidney transplant recipients. Indian J Nephrol. 2024;34:648-51.
- [Google Scholar]
- High-level viruria as a screening tool for BK virus nephropathy in renal transplant recipients. Kidney Res Clin Pract. 2016;35:176-81.
- [CrossRef] [PubMed] [Google Scholar]
- Cost-efficient screening for BK virus in pediatric kidney transplantation: A single-center experience and review of the literature. Pediatr Transplant. 2010;14:589-95.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of and risk factors for BK polyomavirus replication and nephropathy in pediatric renal transplant recipients: An International CERTAIN registry study. Transplantation. 2019;103:1224-1233.
- [PubMed] [Google Scholar]
- BK polyomavirus in solid organ transplantation-guidelines from the american society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33:e13528.
- [PubMed] [Google Scholar]
- BK polyomavirus infection after renal transplantation: Surveillance in a resource-challenged setting. Transpl Infect Dis. 2017;19
- [Google Scholar]
- KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1-155.
- [Google Scholar]






