Translate this page into:
Oral Nutrition Supplementation in the Health Dynamics of Dialysis Patients
Corresponding author: K Merina Elizabeth Joseph, Department of Nutrition and Dietetics, The Madras Medical Mission, Chennai, Tamil Nadu, India, Meenakshi Academy of Higher Education and Research (MAHER), Chennai, Tamil Nadu, India. E-mail: merinaelizalex@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Joseph KME, Dhanavel A, Abraham G, Shankar B. Oral Nutrition Supplementation in the Health Dynamics of Dialysis Patients. Indian J Nephrol. doi: 10.25259/IJN_662_2024
Abstract
Inadequate nutritional management poses health challenges to patients on dialysis. Its iatrogenic effects include intradialytic hypotension, gastrointestinal intolerance, muscle cramps, inflammations, renal osteodystrophy, poor quality of life, and protein energy wasting (PEW). Nutrition care helps manage these effects. Nutritional intervention for these patients includes dietary counseling with food modifications. Oral nutritional supplements (ONS) are recommended when kitchen-based food does not meet nutritional needs. ONS improve the nutrition status, blood parameters (serum albumin, pre-albumin), sleep quality index, hand grip strength, body mass index, nutritional intake, muscle mass, and physical and mental quality of patients on dialysis. ONS types that have shown improvement are soya protein, whey protein, collagen peptide, egg albumin, branched-chain amino acid, a blend of casein, and whey. Enhancing protein digestion by using proteolytic enzymes with a high-protein diet would also help in PEW treatment. Ghrelins also improve the appetite and food intake. The nutrition supplements recommended to dialysis patients need to be used appropriately to suit patient-specific requirements and challenges. A multidisciplinary team and holistic approach are important for better patient outcomes.
Keywords
Dialysis
Nutrition status
Oral Nutrition Supplements
Protein Energy Wasting
Proteolytic enzyme
Introduction
Malnutrition, commonly seen among dialysis patients, is associated with increased mortality and morbidity. Medical nutritional therapy (MNT) is integral in controlling and managing dialysis-associated complications. These complications, namely, intradialytic hypotension, muscle cramps, protein-energy wasting (PEW), gastrointestinal intolerance, inflammation, cardiac diseases, and bone disease [Figure 1], could be controlled through proper diet and lifestyle.1 When the former cannot be maintained by regular kitchen-based food, oral nutrition supplements (ONS) become essential.2 There are many ONS types for dialysis patients. Complete nutritional assessment is necessary to decide the supplement type and amount. Adequate nutritional intake and holistic lifestyle modification can protect patients on hemodialysis (HD) from malnutrition and complications.
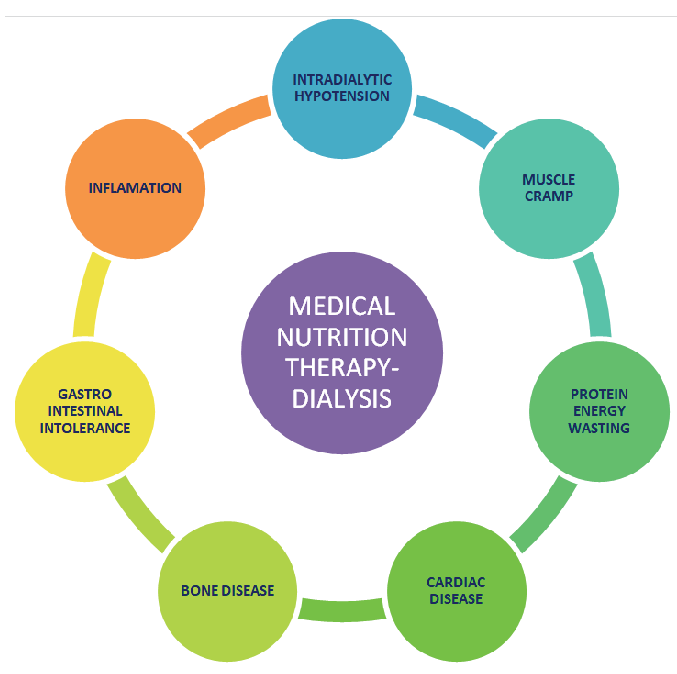
- Significance of medical nutrition therapy in dialysis-associated complication.
Significance of Medical Nutrition Therapy in Dialysis Associated Complication
Intra-dialytic hypotension
The National Kidney Foundation recommends an ideal intradialytic weight gain of 1.5-2 kg. An improper diet with excess salt and fluid could primarily lead to excess intradialytic weight gain. A higher ultrafiltration rate to remove this excess fluid may not be tolerated by some patients, as the rapid fluid removal may cause low blood pressure (hypotension) and cramping. This could lead to inappropriate fluid removal.3 Dietary adherence to fluid and salt restriction needs to be followed to avoid complications. Sugary and salty food causes more thirst. Sodium in the diet should be <2 g per day. The patient’s water requirement varies by urine output.
The dietary energy and protein intake are less on dialysis days.4 Providing a meal or ONS during an HD session can reduce PEW. However, these meals should be individualized according to the patient’s nutritional requirements and risk profile for developing hypotension.5 High-protein snacks or ONS, rather than a full meal with high-carbohydrates, prevent intradialytic hypotension.
Muscle cramps
Muscle cramps among HD patients are multifactorial. Excess fluid removal during dialysis (as stated above), alternation of blood pH (metabolic acidosis/alkalosis), electrolyte imbalance, and carnitine deficiency are common causes.
Metabolic acidosis
Dialysis patients are prone to metabolic acidosis. It is characterized by low serum bicarbonate levels (<22 mmols/L). Dietary management plays a vital role in metabolic acidosis prevention and correction. Including plant-based food rich in alkalis can mitigate metabolic acidosis.6 It is important to include adequate low-potassium vegetables and fruits to mitigate the acidic effects of animal protein consumed to meet high-protein requirements.
Metabolic alkalosis
Metabolic alkalosis due to low protein intake and greater dialysis doses also causes muscle cramps (bicarbonate levels ≥ 26 meq/L) in dialysis patients.7 It is essential to monitor the protein intake of patients on dialysis.
Carnitine deficiency
Carnitine is lost, less produced by the kidneys, or consumed below the requirements, during dialysis, commonly causing deficiency. However, L-carnitine supplementation is warranted only for symptomatic patients when usual measures are unresponsive. Its therapeutic usage has been beneficial for intradialytic muscle cramps, hypotension, asthenia, cardiomyopathy, lowered ejection fraction, muscle weakness or myopathy, reduced oxygen consumption, and anemia requiring large erythropoietin dosage.8 ONS suggested for patients on dialysis are enriched with L-carnitine to meet these specific requirements.
Electrolyte imbalance
Hyperkalemia (Serum potassium > 5 mmol/L) is commonly seen among dialysis patients. Electrolyte imbalance could trigger heart muscle weakening, leading to cardiac arrhythmias. These patients should have a limited dietary potassium intake (40-70 mEq/day).9
Cardiovascular complications
Dietary modifications significantly help curtail cardiovascular complications in patients on dialysis. A meta-analysis confirms improved diastolic pressure (p value = 0.0001), triglycerides (p value = 0.01), and myeloperoxidase (p value = 0.0001) with consumption of plant-based diets rich in polyphenols.10 Supplementation of fermentable soluble fiber improved the lipid profile, oxidative, and inflammatory status in patients on HD.11
Protein-energy wasting
Protein and fat wasting causes weakness and poor quality of life. A 11.95 ± 0.69 g amino acid (AAs) loss was observed per HD session.12 This AA-loss coupled with inadequate food intake, can worsen muscle and fat wasting. Therefore, it becomes essential to ensure adequate protein (1.0 g-1.2 g/kg/day) and energy intake (30-35 kcal/kg ideal body weight) in dialysis patients.13 Protein powders and medium chain triglycerides can be added to food to enhance its protein and energy content.
Renal osteodystrophy
As kidney functions decline, hyperphosphatemia (Serum phosphorus > 4.5 mg/dL) occurs. Most high protein food sources are high in phosphorus. The phosphorus in diet should be < 800-1000 mg per day.14 The desirable phosphorus-to-protein ratio is <12 mg/g of protein.15 ONS are formulated with low phosphorus and high protein.
Gastrointestinal symptoms
Inadequate food intake may be caused due to gastroesophageal reflux disease, gastric intolerance, gastrointestinal inflammation, bloating, constipation, and dyspepsia. Studies have suggested a higher dyspeptic symptom prevalence in patient on HD when compared to the general population. It is defined as an upper abdominal pain or discomfort, bloating, early satiety, post prandial fullness, nausea with or without vomiting, anorexia, regurgitation, and belching.16
Constipation: Constipation is prevalent in 63% HD and 29% of peritoneal dialysis (PD) patients. Medications such as phosphate binders, antibiotics, water restrictions, and dietary restrictions on potassium (restrictions of fruits and vegetables) may exacerbate constipation.17 Sufficient whole grains, fiber-rich low-potassium fruits, and vegetables would aid in better bowel movement and help constipation treatment.
A structured resistance exercise program during HD sessions is a safe and effective intervention that helps improve physical performance, nutritional status, quality of life, anabolic response, and muscle strength.18
Intestinal dysbiosis
Intestinal dysbiosis triggers inflammation and cardiac disease. Supplementing probiotics in the diet suppresses systemic inflammation.19
Food intolerances
Consuming easily digestible foods and improving chew count would aid in better tolerance and digestion. High-fat food remains in the stomach longer, increasing the chances of nausea and vomiting. Small frequent meals also aid in better food tolerance. Cultural and dietary habits vary in different regions of India and this should be factored while advising the diet.
Bloating and indigestion
There is protein dysregulation in dialysis patients. Protein digestion is compromised by dysregulation in its intake.20 Supplementing protein-digesting enzymes aids digestion. Hence, a proteolytic enzyme with protein supplements or protein-rich food would improve its digestion, absorption, and assimilation.21 Ghrelin administrations in dialysis patients have improved appetite and food intake, thereby improving nutritional status.22
Depression and financial limitations commonly seen among dialysis patients can curtail food intake.23 Hence, a holistic approach to improve their nutritional status is needed.
Benefits of oral nutrition supplementation in dialysis patients
Oral nutrition supplementation and dietary counseling significantly improves the nutritional status of patients on dialysis [Figure 2]. This improvement is reflected by parameters like serum albumin, pre-albumin, subjective global assessment (SGA) scores,24 body composition (muscle mass),25 Pittsburgh Sleep Quality Index (PSQI) score,26 physical activity, quality of life,27 body mass index, hand grip strength,28 and malnutrition inflammation score.29
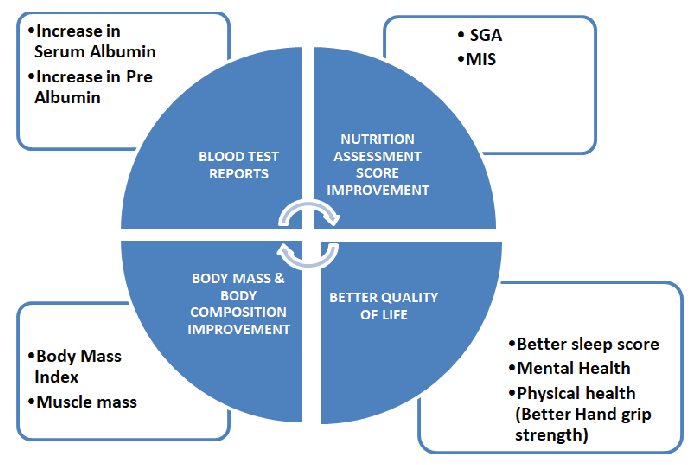
- ONS in MNT of dialysis patients. ONS: Oral nutrition supplement, MNT: Medical nutrition therapy, SGA: Subjective global assessment, MIS: Malnutrition inflammation score.
Oral nutrition supplement in MNT of dialysis patients
Proteins are primarily used as ONS in healthcare settings and gymnasiums. Dietary protein sources, quality, and quantity among different Indian populations have been represented in Table 1.30
| Sex | Food source | Intake (g/day) | Protein content (g/day) | Lysine (mg/g protein) | Digestibility (%) | PDCAAS |
|---|---|---|---|---|---|---|
| Rural-NNMB 2006 | ||||||
| Men | Cereals | 363 ± 185 | 31.6 | 31 | 81 | 81 |
| Millets | 55 ± 128 | 5.3 | 35 | |||
| Pulses & legumes | 31 ± 34 | 6.7 | 70 | |||
| Nuts & oil seeds | 16 ± 31 | 2.9 | 37 | |||
| Fish & flesh foods | 27 ± 39 | 5.9 | 81 | |||
| Milk & milk products | 94 ± 123 | 3.0 | 77 | |||
| Women | Cereals | 328 ± 157 | 28.5 | 31 | 80 | 80 |
| Millets | 37 ± 97 | 3.6 | 35 | |||
| Pulses & legumes | 27 ± 31 | 5.6 | 70 | |||
| Nuts & oil seeds | 14 ± 26 | 2.6 | 37 | |||
| Fish & flesh foods | 24 ± 42 | 5.2 | 81 | |||
| Milk & milk products | 80 ± 112 | 2.6 | 77 | |||
| Urban-NNMB 1984 | ||||||
| Men and Women | Cereals & millets | 381 | 35.1 | 33 | 81 | 81 |
| Pulses & legumes | 47 | 10.2 | 70 | |||
| Nuts & oil seeds | 16 | 2.9 | 37 | |||
| Fish & flesh foods | 24 | 5.1 | 81 | |||
| Milk & milk products | 217 | 6.9 | 77 | |||
| Slum-NNMB 1994 | ||||||
| Men and Women | Cereals & millets | 381 | 35.0 | 33 | 80 | 80 |
| Pulses & legumes | 27 | 5.8 | 70 | |||
| Nuts & oil seeds | 13 | 2.4 | 37 | |||
| Fish & flesh foods | 26 | 5.7 | 81 | |||
| Milk & milk products | 75 | 2.4 | 77 | |||
| Tribal-NNMB 2000 | ||||||
| Men | Cereals | 460 ± 239 | 40 | 31 | 79 | 72 |
| Millets | 5 ± 14 | 0.5 | 35 | |||
| Pulses & legumes | 25 ± 31 | 5.4 | 70 | |||
| Nuts & oil seeds | 17 ± 33 | 3.1 | 37 | |||
| Fish & flesh foods | 20 ± 35 | 4.3 | 81 | |||
| Milk & milk products | 28 ± 59 | 0.9 | 77 | |||
| Women | Cereals | 369 ± 169 | 32.1 | 31 | 80 | 76 |
| Millets | 33 ± 113 | 3.2 | 35 | |||
| Pulses & legumes | 20 ± 27 | 4.3 | 70 | |||
| Nuts & oil seeds | 24 ± 30 | 4.4 | 37 | |||
| Fish & flesh foods | 26 ± 34 | 5.6 | 81 | |||
| Milk & milk products | 29 ± 58 | 0.9 | 77 |
PDCAAS: Protein digestibility-corrected amino acid score, NNMB: National nutrition monitoring bureau
Types of oral nutrition supplements and its implications
The ONS types in dialysis patient management have been presented in Table 2.
| Reference | Type of protein and its intervention |
|---|---|
| Paolo Fanti, Reto Asmis, Tammy J. Stephenson, B. Peter Sawaya, Adrian A. Franke, Nephrology Dialysis Transplantation, Volume 21, Issue 8, August 2006 | End-stage renal disease patients on chronic HD with elevated CRP (>10.0 mg/L) were enrolled in this pilot study. The subjects were double-blinded and randomly distributed with a 2: 1 ratio to receive isoflavone-containing soy-based nutritional supplements (soy group) or isoflavone-free milk protein (control group) for 8 weeks. Serum isoflavone, inflammatory markers, and nutrition markers were assessed at baseline and the end of the treatment. Thirty-two subjects were enrolled. Fifteen subjects in the soy group and 10 in the control group completed the study; five dropped out due to acute illness and two due to food intolerance. After intervention, blood isoflavone levels were 5-10 fold higher in the soy group than in the control group P < 0.001. However, the isoflavone levels ranged widely in the soy group. Variation from baseline of the individual serum isoflavone levels (Δ-isoflavone) and CRP displayed a strong inverse correlation in the soy group (R = −0.599, P < 0.02). In addition, Δ-isoflavone correlated positively with the variation of albumin (R = 0.522, P = 0.05) and insulin-like growth factor-1 (R = 0.518, P < 0.05). Group levels of CRP were not statistically different after the intervention, although a trend towards lower levels was noted in the soy group [18.2 (12.7–29.1) mg/L at baseline vs. 9.7 (5.2–20.7) mg/L at week 8; NS] but not in the control group [20.6 (9.2–38.5) vs. 17.6 (9.1–40.7) mg/L]. |
| Sahathevan, S., Se, C-H., Ng, SH., Khor, BH., Chinna, K., Goh, B. L., Gafor, H. A., Bavanandan, S., Ahmad, G., Karupaiah, T. Clinical Nutrition ESPEN 2018: Volume 25 | 126 malnourished CAPD patients (serum albumin <40 g/L, body mass index (BMI) <24 kg/m2) randomized to the intervention group (IG, n = 65) received whey protein supplement powder (27.4 g) given for 6 months plus dietary counseling (DC) while the control group (CG, n = 61) received DC only. Achieved dietary protein intake (DPI) IG 59.5% (p < 0.001), CG 16.2%, dietary energy intake between groups was non-significant (p > 0.05). A higher DPI paralleled significant increases in serum urea (mean Δ: IG = +2.39 ± 4.36 mmol/L, p = 0.002, d = 0.57 vs. CG = -0.39 ± 4.59 mmol/L, p > 0.05, d = 0.07) and normalized protein catabolic rate, nPCR (mean Δ: IG = +0.11 ± 0.14 g/kg/day, p < 0.001, d = 0.63 vs. CG = +0.001 ± 0.17 g/kg/day, p > 0.05, d = 0.09) CG patients had a significant decline in QOL physical component (mean Δ = -6.62 ± 16.63, p = 0.020, d = 0.47). Using changes in nPCR level as a marker of within IG, ‘positive responders’ achieved significant improvement in weight, BMI, skinfold measures and serum urea (all p < 0.05), while such changes within ‘negative responders’ were non-significant (all p > 0.05). |
| Yuvaraj A, Vijayan M, Alex M, Abraham G, Nair S. Hemodialysis International 2016 Jan;20(1):56-62 | Out of 55 chronic kidney disease patients on MHD (37 women, 18 men) aged between 21 and 67 years, 26 patients received high-protein commercial nutritional supplements (a combination of whey and casein protein), whereas 29 patients received high-protein kitchen feeding (HPKF). Every patient had their MIS, 24-hour dietary recall, hand grip, mid-arm circumference, and triceps skin-fold thickness at 0, 3, and 6 months. MIS, improvement in ONS group 38.1%, HPKF 8.7% (P = 0.04). Improvement in malnutrition in the ONS group, age group >65 years (P = 0.03) and Serum albumin <35 g/L (P = 0.02). Both high-protein kitchen feeding and high-protein commercial nutritional supplement cohorts were observed to have an improvement in overall nutritional status. Older patients >65 years old with lower serum albumin levels (<3.5 g/dL) were observed to have significant improvement in nutritional status with ONS. |
| Gharia SV, Ravichandran P, Periasamy S. International Journal of Nephrology and Therapeutics. 2020; 5(1): 018-024. | 60 ESRD low-income patients on maintenance hemodialysis were included in the study. The candidates were categorized into three groups of 20 each. Baseline demographic, clinical, and data for laboratory assessment of nutritional parameters were collected and repeated at the end of the study (6 weeks). Group 1 received Enzotein-Whey protein 15 grams plus Proteolytic enzyme with 70000 HUT (Hemoglobin unit tyrosine); Group 2 -30 gms plain whey protein without proteolytic enzyme, and Group 3 control group with no supplements. The intervention Groups 1 and 2 showed improvement in their dry weight by 1.6 kg and 1.4 kg, respectively. The post-dialysis recovery time and serum C Reactive Protein (CRP) levels in groups 1 and 2 had declined, which was significant in Group 1 compared to Group 3 (p < 0.05). Clinical improvement in the mid-arm circumference, Triceps fold thickness, hand grip strength, and MIS were observed in Group 1 and Group 2 but were not statistically significant. |
| Fitschen, P. J., Biruete, A., Jeong, J., Wilund, K. R. Hemodialysis International 2017, 21(1):107-116 | MHD patients were recruited and assigned to either daily supplementation with HMB (n = 16) or placebo (n = 17) for 6 months. Measurements of body composition, bone density, strength, physical function, fall risk, quality of life, and blood parameters were measured at baseline and 6 months. Blood was drawn at baseline, 3, and 6 months to measure compliance. No significant effects of HMB on body composition, bone density, strength, physical function, fall risk, quality of life, or blood parameters were observed. On analysis of plasma HMB concentrations, 5 of 16 patients (31%) in the HMB group were found to be noncompliant at 3 or 6 months. Therefore, we performed a per-protocol analysis with compliant participants only and observed no significant differences in our outcomes of interest. |
| González-Espinoza, L., Gutiérrez-Chávez, J., del Campo, F. M., Martínez-Ramírez, H. R., Cortés-Sanabria, L., Rojas-Campos, E., Cueto-Manzano, A. M. Peritoneal Dialysis International. 2005: 25(2):173-80. | 28 CAPD patients were allocated to a study (n = 13) or a control (n = 15) group. Both groups received conventional nutritional counseling; the study group received, additionally, an oral egg albumin-based supplement. During a 6-month follow-up, all patients had monthly clinical and biochemical evaluations and quarterly assessments of the adequacy of dialysis and nutrition. Serum albumin levels were not different between groups; however, a significant increase (baseline vs. final) was observed in the study group (2.64+/-0.35 vs. 3.05+/-0.72 g/dL) but not in the control group (2.66+/-0.56 vs. 2.80+/-0.54 mg/dL). Calorie and protein intake increased more in the study group (calories 1331+/-432 vs. 1872+/-698 kcal; proteins 1.0+/-0.3 vs. 1.7+/-0.7 g/kg) than in the control group (calories 1423+/-410 vs. 1567+/-381 kcal; proteins 1.0+/-0.4 vs. 1.0+/-0.3 g/kg). Similarly, the non-protein nitrogen appearance rate (nPNA) increased significantly more in the study (1.00+/-0.23 vs. 1.18+/-0.35 g/kg/day) than in the control group (0.91+/-0.11 vs. 0.97+/-0.14 g/kg/ day). Triceps skinfold thickness (TSF) and mid-arm muscle area (MAMA) displayed a non-significant trend to a greater increase in the study group (TSF 16.7+/-8.7 vs. 18.3+/-10.7 mm; MAMA 23.8+/-6.2 vs. 25.8+/-5.9 cm2) than in controls (TSF 16.4+/-5.7 vs. 16.9+/-7.0 mm; MAMA 28.7+/-7.8 vs. 30.0+/-7.9 cm2). At the end of the follow-up, the frequency of patients with moderate or severe malnutrition decreased by 6% in the control group and decreased by 28% in the study group. |
| Usamah, A., Mushahar, Govin, S. M., Lim, Y., and Leow, C. W. Kidney International Reports 2019: 4, S1–S437 | 38 individuals were randomized into 2 groups. Group 1 (n=19) received nutritional counseling and oral collagen peptides supplement, while Group 2(n=19) received nutritional counseling only. Nutritional assessments were performed at 0 and 8 weeks using diet records and biochemical and anthropometric measurements: mid-arm muscle circumference (MAMC) and triceps skinfold thickness (TST). There was no significant difference in demographic data, biochemical, or anthropometric measurements between the 2 groups at baseline. At the end of the study period, protein intake had increased from 0.8 to 1.0 g/kg/day (p=0.05) and 0.8 to 0.9 g/kg/day (p=0.02) in Groups 1 and 2, respectively. There was no significant difference in calorie intake between both groups. Serum urea significantly increased in Group 1 from baseline (12.9 to 15.7 mmol/L, p=0.003) vs. Group 2 (12.2 to 12.4 mmol/L, p=0.81). Serum creatinine increased in Group 1 but not Group 2 (p=NS). No significant difference was seen in kt/v in both groups. Serum phosphate had declined from baseline in Group 1 (1.44 to 1.28 mmol/L, p=0.16) but increased in Group 2 (1.49 to 1.56 mmol/l, p=0.06). In Group 2, the increased serum phosphate correlates with their higher phosphate intake (p=0.03). There was no significant improvement in serum albumin levels in both groups. In Group 1, there was an increase in MAMC (27 to 28cm, p=0.29) and TST (12.8 to 14.5mm, p=0.44). However, in Group 2, there was a decline in MAMC (27 to 26.4cm, p=0.25) and TST (16.0 to 14.0 mm, p=1.0) |
| Hiroshige K, Sonta T, Suda T, Kanegae K, Ohtani A. 2001. Nephrology Dialysis Transplant 2001: 16(9):1856-62 | 44 elderly (age >70 years) patients on chronic HD and 28 patients with low plasma albumin concentration (<3.5 g/dL) were classified as the malnourished group; they also suffered from anorexia. The other 16 patients did not complain of anorexia and were classified as the well-nourished group. Fourteen patients each received daily oral BCAA supplementation (12 g/day) or a placebo in random order in a crossover trial for 6 months. Body fat percentage, lean body mass, plasma albumin concentration, dietary protein and caloric intake, and plasma amino acid profiles were monitored. Lower plasma levels of BCAA and lower protein and caloric intakes were found in the malnourished group as compared to the well-nourished group. In BCAA-treated malnourished patients, anorexia and poor oral protein and caloric intake improved within a month concomitant with the improvement in plasma BCAA levels over the values in well-nourished patients. After 6 months of BCAA supplementation, anthropometric indices showed a statistically significant increase, and mean plasma albumin concentration increased from 3.31 g/dL to 3.93 g/dL. After exchanging BCAA for a placebo, spontaneous oral food intake decreased, but the favorable nutritional status persisted for the next 6 months. In 14 patients initially treated with a placebo, no significant changes in nutritional parameters were observed during the first 6 months. However, positive results were obtained by BCAA supplementation during the subsequent 6 months, and mean plasma albumin concentration increased from 3.27 g/dL to 3.81 g/dL. |
Vegetable protein powder
Plant-based diets reduce the obesity, high blood pressure, and diabetes risk.31 A study on patients supplemented with soy protein found an inverse relationship between inflammatory marker CRP levels and serum isoflavone levels (P < 0.02) and positive correlation between Δ-isoflavone and albumin variation (R = 0.522, P = 0.05) and insulin-like growth factor-1 (R = 0.518, P < 0.05).32
Whey protein
Whey protein isolates used in patients on PD showed improved dietary protein intake and increased serum urea (P = 0.002) and normalized Protein catabolic rate (n PCR) (P < 0.001). The body weights, BMIs, skin-fold measures, and serum urea of positive responders increased.33
Balanced tube feeding ONS
The protein sources are a combination of whey and casein protein. ONS given to dialysis patients decreased MIS scores in 38% (p value 0.04) and improved serum albumin (p value = 0.02).34
High protein diet with proteolytic enzyme
Proteolytic enzymes are used to improve protein digestion. Using proteolytic enzymes with a protein-rich diet was beneficial in dialysis patients for better protein assimilation thereby reducing PEW. There was a significant improvement in dry weight and CRP reduction (p value < 0.05).35
Protein supplement with hydroxymethylbutyrate (HMB) helps in muscle protein synthesis by stimulating the mammalian target of rapamycin.36 However, no significant HMB effects on dialysis patients with regard to body composition, bone density, strength, physical function, fall risk, quality of life, or blood parameters were seen.37
Egg albumin protein
Egg albumin-based supplement significantly improved serum albumin, n(PNA), triceps skinfold, mid-arm muscle circumference (MAMC), and calorie protein intake compared with the controls who received diet counseling only (p value < 0.05). The malnutrition percentage reduced by 28% in the study group compared with the control group (6%).38
Collagen peptides
Administration of oral collagen peptide was beneficial in PD patients. It significantly increased protein intake (p value = 0.05) and serum urea levels (p value = 0.003). However, there was no significant serum albumin improvement.39
Branched chain amino acid (BCAA) level was low in malnourished HD patients. Supplementing BCAAs significantly improved the serum albumin, plasma BCAA, oral calorie and protein intake, and anthropometric indices (p value < 0.05).40
Factors considered while recommending ONS
ONS are recommended for malnourished patients or those not meeting nutritional requirements. Malnutrition could be diagnosed using nutrition assessment tools like malnutrition inflammation score (MIS)41 and SGA.42
Malnourished patients are recommended to take ONS twice or thrice a day based on their nutrition deficit. Consumption is recommended between the main meals to avoid interference with normal kitchen-based food quantity. Proteolytic enzymes are advised immediately after a high-protein meal. The ONS recommended for patients on dialysis should adhere to fluid, sodium, potassium, and phosphorus restrictions. ONS with the lowest phosphorus-to-protein ratio (< 12) are preferred. ONS with additional benefits on fiber, probiotics, and carnitine are preferred. Protein from egg, whey, BCAA, and collagen can be considered.
A balanced oral nutrition formula is recommended for reduction in overall intake. The recommended guidelines for patients include: total fat ≤ 30% of energy intake, reducing saturated fat (<10% of total energy), monounsaturated fat (≤20% of total energy), polyunsaturated fat (≤10% of total energy), and cholesterol intake (<200 mg/d). Daily carbohydrate intake should reach 45% to 65% total energy and fiber intake ≥20 g/d.43
Proteins with neutral flavors are available. These types are used when patients resist commercial preparations with different flavors. Patients prefer routine food without flavor. It could be mixed into food. Micronutrient supplementation should be individualized based on nutrient levels indicated by blood test investigation. Formulas with high in Vitamin A & E should be avoided as they could be toxic.44
Vegetable-source protein supplements could be recommended for patients having high phosphorus values (>4.5 mg/dL), as the phosphorus in vegetable protein is less bioavailable. The taste and flavor preferences of the patient should also be considered while planning ONS.
ONS mixing & Administration: ONS should be mixed in lukewarm water for better miscibility. Medications should not be mixed in ONS as they can interfere with nutrient absorption.
Economic constraints of patients: The patients’ financial constraints and consent should be considered before planning ONS. Kitchen-based protein-rich snacks could be included in the diet of patients having financial constraints.
Dialysis-associated complications should be efficiently managed by implementing the appropriate nutritional intervention. Dietary modification and suggesting suitable ONS as per guidelines would safeguard patients from malnutrition and provide a better quality of life.
Conflicts of interest
There are no conflicts of interest.
References
- Assessment and management of nutrition in hemodialysis patients. J Renal Nutr Meta. 2020;5:83-7.
- [Google Scholar]
- Effects of oral nutritional supplements on the nutritional status and inflammatory markers in patients on maintenance dialysis: A systematic review and meta-analysis of randomized clinical trials. Clin Kidney J. 2023;16:2271-88.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevention of intradialytic hypotension in hemodialysis patients: Current challenges and future prospects. Int J Nephrol Renovasc Dis. 2023;16:173-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of dietary intake, appetite, and eating habits on dialysis and non-dialysis treatment days in hemodialysis patients: Cross-sectional results from the HEMO study. J Ren Nutr. 2003;13:191-8.
- [CrossRef] [PubMed] [Google Scholar]
- Eating during the hemodialysis session: A practice improving nutritional status or a risk factor for intradialytic hypotension and reduced dialysis adequacy? Nutrients. 2020;12:1703.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Nutritional approaches for the management of metabolic acidosis in chronic kidney disease. Nutrients. 2021;13:2534.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The pathophysiology of leg cramping during dialysis and the use of carnitine in its treatment. Physiol Rep. 2021;9:e15114.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Nutritional requirements in maintenance hemodialysis. Adv Ren Replace Ther. 2003;10:183-93.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of polyphenol-rich interventions on cardiovascular risk factors in haemodialysis: A systematic review and meta-analysis. Nutrients. 2017;9:1345.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of fermentable dietary fiber supplementation on oxidative and inflammatory status in hemodialysis patients. Int J Clin Exp Med. 2015;8:1363-9.
- [PubMed] [PubMed Central] [Google Scholar]
- End-stage renal disease patients lose a substantial amount of amino acids during hemodialysis. J Nutr. 2020;150:1160-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76:S1-S107.
- [CrossRef] [PubMed] [Google Scholar]
- The importance of nutritional intervention by dietitians for hyperphosphatemia in maintained hemodialysis patients. Ren Replace Ther. 2017;3
- [CrossRef] [Google Scholar]
- The “phosphorus pyramid”: A visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 2015;16:9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dyspepsia amongst end stage renal disease undergoing hemodialysis: Views from a large tertiary care center. J Transl Int Med. 2018;6:78-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am J Kidney Dis. 2002;39:1292-9.
- [CrossRef] [PubMed] [Google Scholar]
- Benefits of physical exercise in patients with chronic kidney disease and hemodialysis: A mini review. UNOAJ. 2017;5
- [Google Scholar]
- The effect of probiotic supplementation on systemic inflammation in dialysis patients. Kidney Res Clin Pract. 2022;41:89-101.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dysregulated handling of dietary protein and muscle protein synthesis after mixed-meal ingestion in maintenance hemodialysis patients. Kidney Int Rep. 2018;3:1403-15.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Combating protein energy wasting in end stage kidney disease: Role of exogenous proteolytic enzyme as an adjuvant to dietary protein. Int J Basic Clin Pharmacol. 2019;8:1424.
- [CrossRef] [Google Scholar]
- Sustained appetite improvement in malnourished dialysis patients by daily ghrelin treatment. Kidney Int. 2009;76:199-206.
- [CrossRef] [PubMed] [Google Scholar]
- Depressive symptoms and dietary non-adherence among end stage renal disease patients undergoing hemodialysis therapy: Systematic review. BMC Nephrol. 2019;20:429.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Therapeutic effects of oral nutritional supplementation during hemodialysis. Kidney Int. 2002;62:1054-9.
- [CrossRef] [PubMed] [Google Scholar]
- Muscle status response to oral nutritional supplementation in hemodialysis patients with protein energy wasting: A multi-center randomized, open label-controlled trial. Front Nutr. 2021;8:743324.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sleep quality after intradialytic oral nutrition: A new benefit of this anabolic strategy? A pilot study. Front Nutr. 2022;9:882367.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Intradialytic nutrition and quality of life in chilean older patients in hemodialysis with protein-energy wasting. Int Urol Nephrol. 2022;54:1947-55.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of oral nutritional supplements on the nutritional status and inflammatory markers in patients on maintenance dialysis: A systematic review and meta-analysis of randomized clinical trials. Clin Kidney J. 2023;16:2271-88.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Intradialytic oral nutrition effects on malnourished hemodialysis patients: A randomized trial. Sci Rep. 2024;14
- [CrossRef] [Google Scholar]
- Positive effect of dietary soy in ESRD patients with systemic inflammation—correlation between blood levels of the soy isoflavones and the acute-phase reactants. Nephrol Dial Transplant. 2006;21:2239-46.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical efficacy and feasibility of whey protein isolates supplementation in malnourished peritoneal dialysis patients: A multicenter, parallel, open-label randomized controlled trial. Clin Nutr ESPEN. 2018;25:68-77.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of high-protein supplemental therapy on subjective global assessment of CKD-5D patients. Hemodial Int. 2016;20:56-62.
- [CrossRef] [PubMed] [Google Scholar]
- Can ENZOTEIN (proteolytic enzyme fortified protein supplement) be an effective alternative as low dose protein supplement in malnourished low income end stage renal disease patients? Int J Nephrol Ther. 2020;5:18-24.
- [Google Scholar]
- Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle. 2017;8:529-41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy of beta-hydroxy-beta-methylbutyrate supplementation in maintenance hemodialysis patients. Hemodial Int. 2017;21:107-16.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized, open label, controlled clinical trial of oral administration of an egg albumin-based protein supplement to patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 2005;25:173-80.
- [PubMed] [Google Scholar]
- Sun-082 Effects of oral collagen peptides on nutritional status of peritoneal dialysis patients. Kidney International Reports. 2019;4:S188-9.
- [CrossRef] [Google Scholar]
- Oral supplementation of branched-chain amino acid improves nutritional status in elderly patients on chronic haemodialysis. Nephrol Dial Transplant. 2001;16:1856-62.
- [CrossRef] [PubMed] [Google Scholar]
- The association malnutrition-inflammation score with chronic kidney disease-associated pruritus and quality of life in hemodialysis patients: A multicenter cross-sectional study. Sci Rep. 2024;14
- [CrossRef] [Google Scholar]
- A modified quantitative subjective global assessment of nutrition for dialysis patients. Nephrol Dial Transplant. 1999;14:1732-8.
- [CrossRef] [PubMed] [Google Scholar]
- Associations of dietary macronutrients and micronutrients with the traditional and nontraditional risk factors for cardiovascular disease among hemodialysis patients: A clinical cross-sectional study. Medicine (Baltimore). 2018;97:e11306.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Vitamin supplement use in patients with CKD: Worth the pill burden? Am J Kidney Dis. 2024;83:370-85.
- [CrossRef] [PubMed] [Google Scholar]







