Translate this page into:
Outcomes of Endovascular Treatment for Salvaging Failed Hemodialysis Arteriovenous Fistula – Role of Balloon Angioplasty as Initial Therapy
Corresponding author: S. Vignesh, Department of Imaging Sciences and Interventional Radiology, Meenakshi Mission Hospital Research Centre, Madurai, Tamil Nadu, India. E-mail: vigneshmdrd@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vignesh S, Mukuntharajan T, Sampathkumar K. Outcomes of Endovascular Treatment for Salvaging Failed Hemodialysis Arteriovenous Fistula – Role of Balloon Angioplasty as Initial Therapy. Indian J Nephrol. 2024;34:583-8. doi: 10.25259/ijn_539_23
Abstract
Background:
This study aims to evaluate the technical and clinical outcomes of endovascular treatment for failed native hemodialysis fistulas, mainly the role of balloon angioplasty in salvaging thrombosed and stenosed arteriovenous fistulas.
Materials and Methods:
This retrospective study was done on 23 patients who had presented with non-functioning dialysis fistulas. The mean age of the patients was 58.7 ± 2.3 years. The cause of failure was thrombosis in 14 cases (61%) and stenosis in 9 cases (39%). All patients initially underwent percutaneous transluminal angioplasty (PTA), followed by thromboaspiration depending on the thrombus load and extent.
Results:
A total of 27 salvage procedures were performed on 23 patients. Technical success was achieved in 24 procedures (88.8%), and clinical success was 81.5%. Patients were followed up for mean duration of 9.5 months (range: 1–19 months). Eight out of 23 accesses initially revised failed again due to repeat thrombosis, of which four patients underwent repeat procedures. The mean duration to re-intervention was 5.5 ± 1.3 months. The primary patency rates were 79% at 3 months and 60% at 6, 12, and 18 months. The cumulative (secondary) patency rates were 73% at 6 months and 66% at 12 and 18 months. Minor complications were seen in three procedures (11%), which included venous extravasation in two cases and prolonged bleeding from puncture site in one case.
Conclusion:
Percutaneous balloon angioplasty can be used as first-line procedure in failed hemodialysis fistulas, in both cases of stenosis and/or thrombosis, followed by thromboaspiration if required.
Keywords
Hemodialysis arteriovenous fistula
Failed hemodialysis fistula
Thrombosed fistula
Salvaging non-functional fistula
Percutaneous transluminal angioplasty
Endovascular management
Introduction
Native arteriovenous fistula (AVF) remains the vascular access of choice for maintenance hemodialysis according to Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines for hemodialysis vascular access.1 Access-related complications lead to patient morbidity and reduced quality of life. Fistula stenosis and thrombosis remain the main causes of both early and delayed failure of AVFs, leading up to 65–85% of cases of permanent access loss.2 Such patients require either new AVF creation or placement of central venous catheters.
The main factor leading to stenosis is endothelial cell injury resulting in smooth muscle proliferation and neointimal hyperplasia.3 Thrombosis of AVF can occur due to many causes, the most common being stasis due to stenoses, either in inflow arterial, juxta-anastomotic, or outflow venous sites. Systemic factors such as hypotension or hypercoagulability (common in end-stage kidney disease patients) and local factors including prolonged compression on the puncture site post dialysis or percutaneous procedures, and improper needling techniques also lead to increased risk of thrombosis.2,4 Lack of bruit across AVF, swelling of the arm, or erythema points toward the possibility of dysfunctional AVF. Ultrasound (USG) may show the level and extent of stenosis and the presence of thrombus. Computed tomography venography or percutaneous venography may be required when thrombosis or stenosis of intrathoracic central veins are suspected.4
Treatment of failed fistulas is required to ensure long-term survival and improve patients’ quality of life. Percutaneous transluminal angioplasty (PTA) is now an established method of treatment for stenosis and/or thrombosis associated with stenosis.5,6 Other endovascular methods available for salvaging the thrombosed fistulas include catheter directed/direct percutaneous thrombolysis or mechanical thrombectomy such as balloon angioplasty to fragment or macerate the thrombus or thromboaspiration.6-9
The purpose of this study is to evaluate the technical and clinical outcomes of endovascular treatment for failed native hemodialysis fistulas, mainly the role of balloon angioplasty in salvaging thrombosed and stenosed AVFs. The primary and secondary patency rates and complications associated with these procedures were also assessed.
Materials and Methods
This was a retrospective single-center study done on 23 patients (21 males, 2 females) from January 2021 to January 2023, who had presented with non-functioning or hypofunctioning dialysis fistulas, as detected clinically by absent/decreased thrill and/or reduced flows and/or increased pressures during dialysis. Infected fistulas were excluded. The mean age of the patients was 58.7 ± 2.3 years (range: 30–76 years). Of 23 patients, 11 (47.8%) had radiocephalic fistulas, 11 (47.8%) had brachiocephalic fistulas, and 1 (4.3%) had brachiobasilic fistula. The average duration of fistula functioning till its failure post-creation was 14.8 ± 2.8 months (range: 7–46 months). All the patients underwent pre-procedural USG to determine the cause for fistula failure and found to be thrombosis in 14 cases (61%) and stenosis without any thrombus in 9 cases (39%). Among 14 thrombosed AVFs, associated stenosis was seen in 12 cases (86%). Written informed consents were obtained from the patients before the endovascular procedures.
Those with stenosis underwent PTA and those with thrombosis with/without underlying stenosis underwent mechanical thrombectomy in the form of PTA initially and thromboaspiration depending.
All the procedures were done in aseptic precautions, under local anesthesia. Retrograde arterial access in the distal radial artery (at wrist level) was preferred in all cases with 6F vascular access sheath (Input Introducer Sheath, Medtronic); unless sufficient length of distal radial artery was not available or radial artery was narrowed/calcified, in which case antegrade or retrograde venous (cephalic) access was placed. Initial fistulogram was obtained which showed the level of obstruction. The stenotic/thrombotic segment was negotiated through 0.035 inch hydrophilic Guidewire (Terumo, Radifocus Terumo Guidewire, Germany) through 5F diagnostic catheter (MPA, RDC catheters, Cook Medical LLC, Bloomington, IN, USA). Angioplasty was performed with PTA balloons of diameters ranging from 4 mm to 8 mm (Advance, Cook Medical LLC, Bloomington, IN, USA; Mustang, Boston Scientific, MA, USA). They were kept inflated for 120–150 s across the level of obstruction. During balloon inflation, analgesia in the form of intravenous tramadol (50–75 mg)/pentazocine (25–50 mg) was given, if the patient complained of pain. Post-dilatation, venography was done to check the response [Figures 1 and 2]. If the response was suboptimal, repeat angioplasty was done. If sufficient clots were still present, thrombolysis was performed by instilling 50,000–1,00,000 IU urokinase or 10 mg alteplase followed by thrombus aspiration using a 6F guiding catheter (Cordis, FL, USA). USG was used as an adjunct to look for the resolution of clots. Adequate anticoagulation was maintained with 50–60 IU/kg heparin as loading dose, followed by 15–20 IU/kg/h as maintenance. Post-procedure, the patient was placed on adequate anticoagulation with intravenous heparin 5000 IU 6 hourly for at least 24 h. Patients who underwent successful hemodialysis post-procedure were followed up at 1, 6, and 12 months.
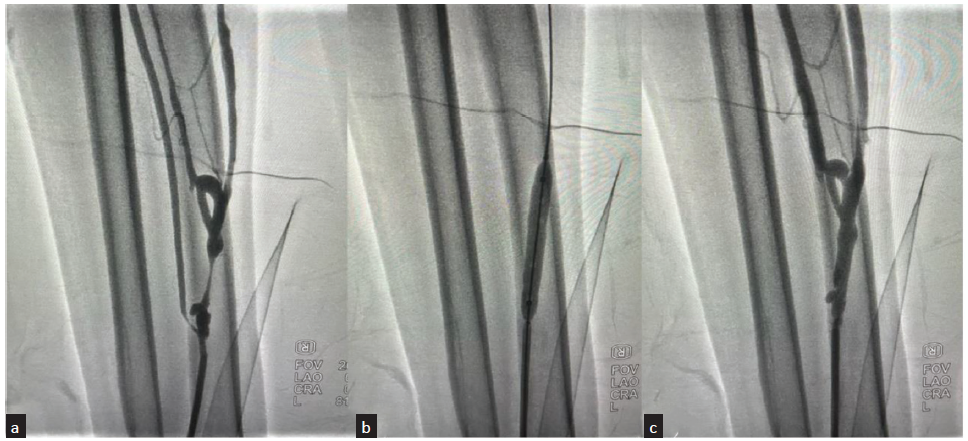
- Angiographic images of percutaneous balloon angioplasty performed for radio-cephalic fistula with stenosis in 53-year-male patient. (a) Fistulogram from retrograde radial access showing short segment stenosis of the draining cephalic vein just distal to fistula; (b) Percutaneous angioplasty performed by inflating the balloon across the stenosis; (c) Post angioplasty, angiography showing no residual stenosis with good flow into the cephalic vein.
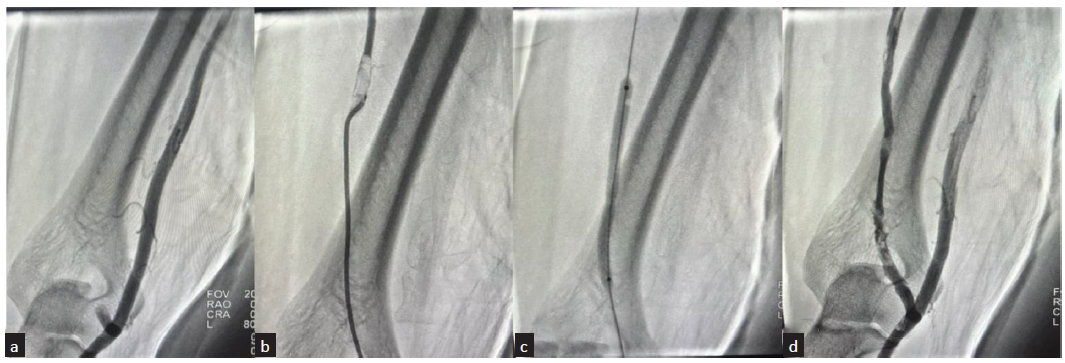
- Angiographic images of percutaneous balloon angioplasty performed for thrombosed brachio-cephalic fistula in 64-year-male patient. (a) Fistulogram from retrograde radial access showing cut-off beyond short stump of cephalic vein at elbow; (b) Catheter negotiated through the thrombotic segment and angiogram showing filling defect in upper cephalic vein suggestive of thrombus; (c) Percutaneous angioplasty performed by inflating the balloon across the thrombotic segment; (d) Post angioplasty, angiography showing good flow across the fistula into the cephalic vein, with no demonstrable filling defects.
Definition
According to the standardized definitions for hemodialysis vascular access published by the North American Vascular Access Consortium (NAVAC), anatomical or technical success is defined as achieving an immediate post-procedure residual stenosis of <30%. Clinical success is the resumption of normal dialysis for at least one session after the procedure.10 As described by the kidney health initiative: Vascular access clinical trial endpoints project, post-intervention primary patency is defined as the time from the index intervention until the repeat access thrombosis or repeat intervention. Cumulative/secondary patency is defined as the time from the index intervention until the access is abandoned with failure of any endovascular technique, necessitating the creation of a new fistula, dialysis graft or catheter insertion.11
All procedures performed in the study involving human participants were per the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) 29.0 statistical software for Windows (SPSS Inc, Chicago, IL, USA). The data are presented as mean standard deviation or the percentage. Kaplan–Meier survival analysis was used to calculate post-intervention patencies.
Results
A total of 27 salvage procedures were performed on 23 patients. All underwent balloon angioplasties as an initial procedure either to relieve stenosis or to mechanically fragment the thrombus. In addition, thrombolysis followed by thromboaspiration was done in eight cases of thrombosed AVFs. Radial access was utilized in 21 procedures (78%), antegrade cephalic venous access in two cases and retrograde cephalic access in three cases. In one procedure, both radial and cephalic access had to be obtained. Technical success was achieved in 24 procedures (88.8%). In one case, the draining cephalic vein had long segment occlusion that could not be traversed, and in two cases, chronic adherent thrombus was noticed which could not be removed. Repeat thrombosis occurred within 1–2 days post-procedure, with clinical success of 81.5%. In other cases, hemodialysis through the fistula was initiated the next day.
After initial procedure, patients were followed up for mean of 9.5 months (range: 1–19 months). Eight out of 23 accesses (35%) initially revised failed again due to repeat thrombosis. Of these 8 cases, 4 patients (50%) underwent repeat procedures in the form of mechanical thrombectomy with aspiration and were functional for mean of 5.7 months. Two patients were converted into peritoneal dialysis and two others required the creation of new fistula. The mean duration from initial procedure to re-intervention was 5.5 ± 1.3 months (range: 1–10.5 months).
The primary patency rates were 79% at 3 months and 60% at 6, 12, and 18 months for those who had technical and clinical success [Figure 3]. The cumulative (secondary) patency rates were 73% at 6 months and 66% at 12 and 18 months [Figure 4]. The patency rates for fistula failures presenting with stenosis were slightly better than those presenting with thrombosis, but they did not reach statistical significance (67% vs. 52% at 12 months; P = 0.038). The patients aged <60 years had better patency rates in comparison with those above 60 years (86% vs. 42%; P = 0.004). Radiocephalic fistulas performed better as compared to above elbow (brachiocephalic and brachiobasilic) fistulas, though statistical significance was not achieved (77% vs. 45% at 12 months; P = 0.360) [Figure 5].
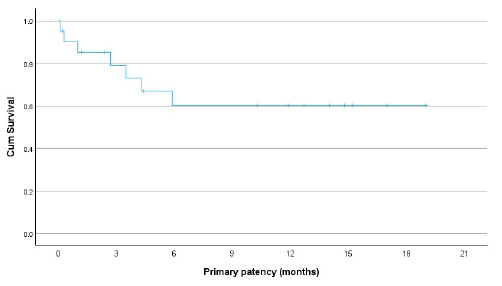
- Graph showing primary patency rates of failed hemodialysis arteriovenous fistulas post endovascular treatment with percutaneous balloon angioplasty as initial therapy.
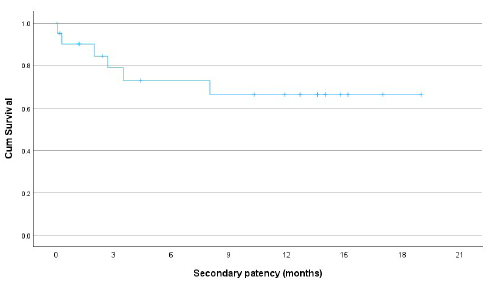
- Graph showing secondary (cumulative) patency rates of failed hemodialysis arteriovenous fistulas post endovascular treatment with percutaneous balloon angioplasty as initial therapy.
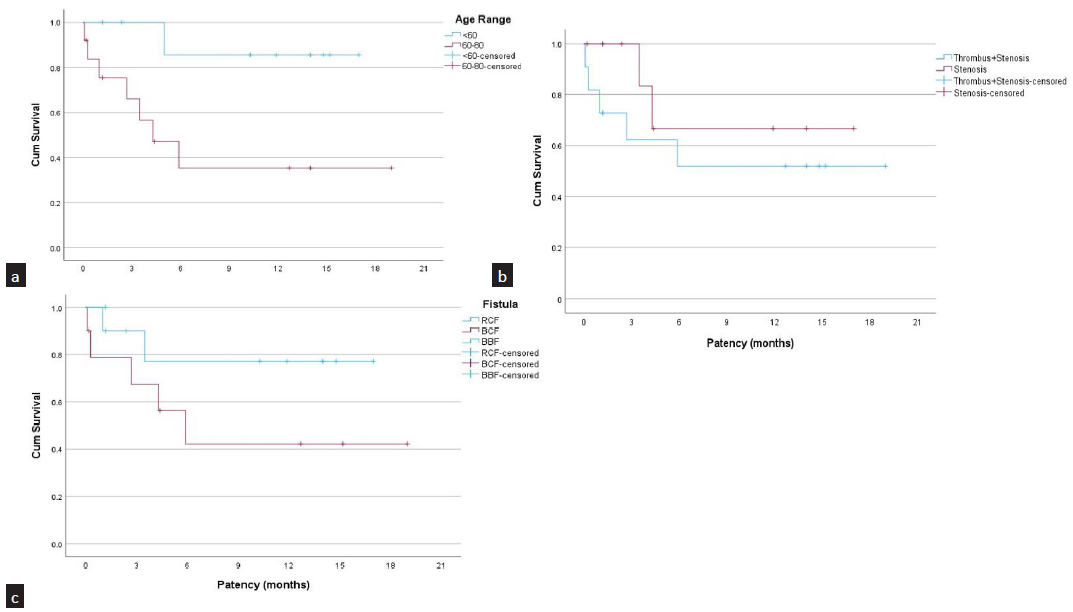
- Graph showing comparison of patencies between various groups. (a) Comparison between patients with age <60 years (blue) and >60 years (red); (b) Comparison between dialysis fistula presenting with thrombosis (blue) and stenosis (red); (c) Comparison between radiocephalic fistulas (blue) and brachiocephalic fistulas (red). RCF: Radiocephalic fistula, BCF: Brachiocephalic fistula, BBF: Brachiobasilic fistula.
Minor complications were seen in 3 procedures (11%), which included venous extravasation in two cases and prolonged bleeding from puncture site in one case. In both cases of venous extravasation, balloon inflation was done at the site to control the leak. There were no major procedure-related complications leading to mortality or increased hospital stay.
Discussion
Failure of dialysis fistulas either due to stenosis or thrombosis should be treated without delay, to limit the progression of thrombosis resulting in loss of precious access and to prevent the need of the central venous catheter. Endothelial cell injury, occurring mainly from mechanical trauma due to venipunctures and shear stress from turbulent blood flow, is the inciting factor in the development of stenosis. The most common site for fistula stenosis is either at the juxta-anastomotic site or outflow vein.3,12,13 Dialysis access thrombosis can be attributed to Virchow’s triad of stasis, hypercoagulability, and endothelial injury/dysfunction.2 Stasis may be due to reduced inflow as can occur with systemic hypotension or decreased outflow as seen with venous stenosis. The most common underlying cause for thromboses is venous stenoses, as was seen in 86% of our thrombosed fistulas.
Stenosis per se can cause the hypofunction of fistulas due to reduced flow in the venous segment or can lead to thrombus formation. It is always imperative to treat the stenosis, even in thrombosed fistulas. The choice of treatment can be either percutaneous balloon angioplasty or open surgical revision. PTA is considered as mainstay in the treatment of stenosis, as it can be rapidly performed and the access can be used for hemodialysis as earliest as the following day. Surgical thrombectomy with revision has a poor outcome with reported success rates ranging from 28% to 73%.5,6,14,15
As opposed to treating stenosis, there is no general consensus regarding management of thrombosed fistulas. Endovascular approach to treating thrombosis can be broadly divided into – (1) pharmacological thrombolysis – usage of thrombolytic agents such as urokinase or recombinant tissue plasminogen activator (alteplase and reteplase); injected either directly into the clot or through catheter-directed;7-9,16 (2) mechanical thrombectomy – mechanical clot disruption performed using direct contact devices (Compliant balloons, Arrow-Trerotola percutaneous thrombolytic device; Arrow International, Reading, PA, USA), hydrodynamic rheolytic devices (AngioJet catheter, Possis Medical, Minneapolis, MN, USA; Amplatz thrombectomy device, Microvena, MN, USA), or percutaneous thromboaspiration;6,17 and (3) pharmacomechanical thrombolysis – combination of both techniques. Each of these techniques has their own advantages; however, the technical success does not vary much. Purely medical thrombolysis can be done only in the initial 24–48 h of thrombus formation. As the clot ages, it adheres to the vessel wall and becomes resistant to any treatment.9,18 The main goal of thrombolytic therapy is to dissolve the clot to promote blood flow across the occluded site. Entire thrombus need not be removed, since with restoration of blood flow across fistula site, the residual thrombus gets dissolved by inherent fibrinolysis.19 Among mechanical thrombectomy devices, except for balloon angioplasty and aspiration, the high cost and availability of other devices preclude their usage in routine settings.
Our study showed that the endovascular approach has 89% technical and 82% clinical success. This is comparable to pharmacomechanical methods employed in other studies, which ranged from 75% to 93%.5,8,9,20-23 The primary patency rates in our study were 79% at 3 months and 60% at 6 and 12 months, and the cumulative patency rates were 73% at 6 months and 66% at 12 months. This is similar to the studies by Miquelin et al. and Tan et al., who performed PTA for failing dialysis fistulas with stenoses and reported patency rates of 81% and 50%, and 72% and 32% at 6 and 12 months, respectively.24,25 The other studies on thrombosed fistulas that utilized combination treatment had varying results. Çildağ et al.17 utilized pharmacomechanical thrombectomy similar to ours; they treated 24 thrombosed AVFs with balloon angioplasty and thromboaspiration and reported primary patency rates of 55% and 40% at 6 and 12 months, and secondary patency rates of 70% at 12 months.17 In another study by Zaleski et al., initial angioplasty followed by urokinase thrombolysis was performed and they reported a technical success rate of 82% with primary patency rates of 71% at 6 months and 64% at 12 months.20 Cho et al. in another variation of this technique by first pulse-spraying urokinase followed by balloon maceration in 14 patients reported a technical success of 77% with primary patency rates of 64% and 55% at 6 and 12 months, respectively.21 Even in our study, when the patency rates were separately calculated for thrombosis, it was found out to be 60% at 6 months and 51% at 12 months. This can be attributed to the fact that thrombosed fistulas in general are more prone for recurrent thrombosis due to endothelial injury.
Minor bleeding complications have been reported with the use of thrombolytic agents upto 10%.18,26 Intraprocedural venous rupture can be seen during PTA in 2–16% of cases, as was seen in two of our patients.24 They were managed conservatively by balloon inflation and did not translate to increased morbidity for patients.
The limitations with this study were smaller study population and inherent selection bias. A larger prospective study is needed to compare the effects of balloon angioplasty thrombectomy with other pharmacomechanical techniques in patients to establish the most appropriate method.
Percutaneous balloon angioplasty can be used as first-line procedure in failed hemodialysis fistulas, in both cases of stenosis and/or thrombosis, followed by thromboaspiration if required. It yields excellent technical and clinical success of 82% with well-acceptable patency rates of 66% at 12 months. PTA is an efficacious technique with a shorter time of operation, cost-effectiveness, and minor complication rates.
Conflicts of interest
There are no conflicts of interest.
References
- KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis. 2020;75:S1-164.
- [CrossRef] [PubMed] [Google Scholar]
- Hemodialysis access thrombosis. Cardiovasc Diagn Ther. 2017;7:S299-308.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dialysis arteriovenous fistula failure and angioplasty: Intimal hyperplasia and other causes of access failure. Am J Kidney Dis. 2017;69:147-51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- arteriovenous access failure, stenosis, and thrombosis. Can J Kidney Health Dis. 2016;3:2054358116669126.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Durability of percutaneous transluminal angioplasty for failing hemodialysis vascular access, Retrospective Cohort Study. Int J Surg Open. 2020;24:185-8.
- [CrossRef] [Google Scholar]
- The radiological management of the thrombosed arteriovenous dialysis fistula. Clin Radiol. 2011;66:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous management of thrombosed dialysis access grafts. Semin Intervent Radiol. 2004;21:77-81.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacomechanical thrombolysis for the treatment of thrombosed native arteriovenous fistula: A Single-Center Experience. Pol J Radiol. 2014;79:363-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Direct Percutaneous Thrombolysis (DPT): A novel method of salvaging thrombosed native arteriovenous fistula. J Vasc Access. 2024;25:1158-1163.
- [CrossRef] [PubMed] [Google Scholar]
- Standardized definitions for hemodialysis vascular access. Semin Dial. 2011;24:515-24.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical trial end points for hemodialysis vascular access: Background, rationale, and definitions. Clin J Am Soc Nephrol. 2018;13:490-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Arteriovenous fistulas and their characteristic sites of stenosis. AJR Am J Roentgenol. 2015;205:726-34.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular access stenosis: Comparison of arteriovenous grafts and fistulas. Am J Kidney Dis. 2004;44:859-65.
- [PubMed] [Google Scholar]
- Transluminal angioplasty versus conventional operation in the treatment of haemodialysis fistula stenosis: Results from a 5-year study. Br J Surg. 1987;74:1004-5.
- [CrossRef] [PubMed] [Google Scholar]
- Primary Access surgery for long-term haemodialysis. Aust N Z J Surg. 1994;64:763-7.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombosed hemodialysis grafts: Lyse and wait with tissue plasminogen activator or urokinase compared to mechanical thrombolysis with the arrow-trerotola percutaneous thrombolytic device. J Vasc Interv Radiol. 2001;12:1157-65.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous treatment of thrombosed hemodialysis arteriovenous fistulas: Use of thromboaspiration and balloon angioplasty. Clujul Med. 2017;90:66-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparative efficacy and safety of local and peripheral venous thrombolytic therapy with urokinase for thrombosed hemodialysis arteriovenous fistulas. Exp Ther Med. 2019;17:4279-84.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hydrodynamic thrombectomy of haemodialysis grafts and fistulae: Results of 51 procedures. Nephrol Dial Transplant. 1996;11:1058-64.
- [CrossRef] [PubMed] [Google Scholar]
- Angioplasty and bolus urokinase infusion for the restoration of function in thrombosed brescia-cimino dialysis fistulas. J Vasc Interv Radiol. 1999;10:129-36.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous treatment of failed native dialysis fistulas: Use of pulse-spray pharmacomechanical thrombolysis as the primary mode of therapy. Korean J Radiol. 2006;7:180-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- procedural success and patency after percutaneous treatment of thrombosed autogenous arteriovenous dialysis fistulas. J Vasc Interv Radiol. 2002;13:1211-8.
- [CrossRef] [PubMed] [Google Scholar]
- Restoration of thrombosed brescia-cimino dialysis fistulas by using percutaneous transluminal angioplasty. Radiology. 2002;223:339-44.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous transluminal angioplasty in the treatment of stenosis of arteriovenous fistulae for hemodialysis. Int Arch Med. 2008;1:16.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of endovascular intervention for salvage of failing hemodialysis access. Ann Vasc Dis. 2011;4:87-92.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Complications associated with the use of urokinase and recombinant tissue plasminogen activator for catheter-directed peripheral arterial and venous thrombolysis. J Vasc Interv Radiol. 2000;11:295-8.
- [CrossRef] [PubMed] [Google Scholar]








