Translate this page into:
Oxidative Stress, DNA Damage, Inflammation and Endothelial Dysfunction in Snakebite-Induced Acute Kidney Injury
Corresponding author: Aparna R Bitla, Department of Biochemistry, Sri Venkateswara Institute of Medical Sciences, Tirupati, India. E-mail: aparnabitla@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Pavuluri LA, Bitla AR, Vishnubotla SK, Rapur R. Oxidative Stress, DNA Damage, Inflammation and Endothelial Dysfunction in Snakebite-Induced Acute Kidney Injury. Indian J Nephrol. 2025;35:349-54. doi: 10.25259/ijn_545_23
Abstract
Background
Snakebite-induced acute kidney injury (SAKI) is a life-threatening complication. Despite its impact on public health, the understanding of the underlying cellular and molecular mechanisms remains limited. There is a lack of studies investigating the role of oxidative stress, oxidative deoxyribonucleic acid (DNA) damage, inflammation, and endothelial dysfunction in SAKI. This study aims to address this knowledge gap.
Materials and Methods
Biomarkers of oxidative stress, including oxidative DNA damage, inflammation, and endothelial dysfunction were assessed in 30 patients with SAKI and 30 healthy controls. Malondialdehyde (MDA), protein carbonyl content (PCC), advanced glycation end products (AGEs), 8-hydroxy-2’-deoxyguanosine (8-OHdG), ferric reducing ability of plasma (FRAP), high-sensitivity C-reactive protein (hs-CRP), and nitric oxide (NO) were used as biomarkers.
Results
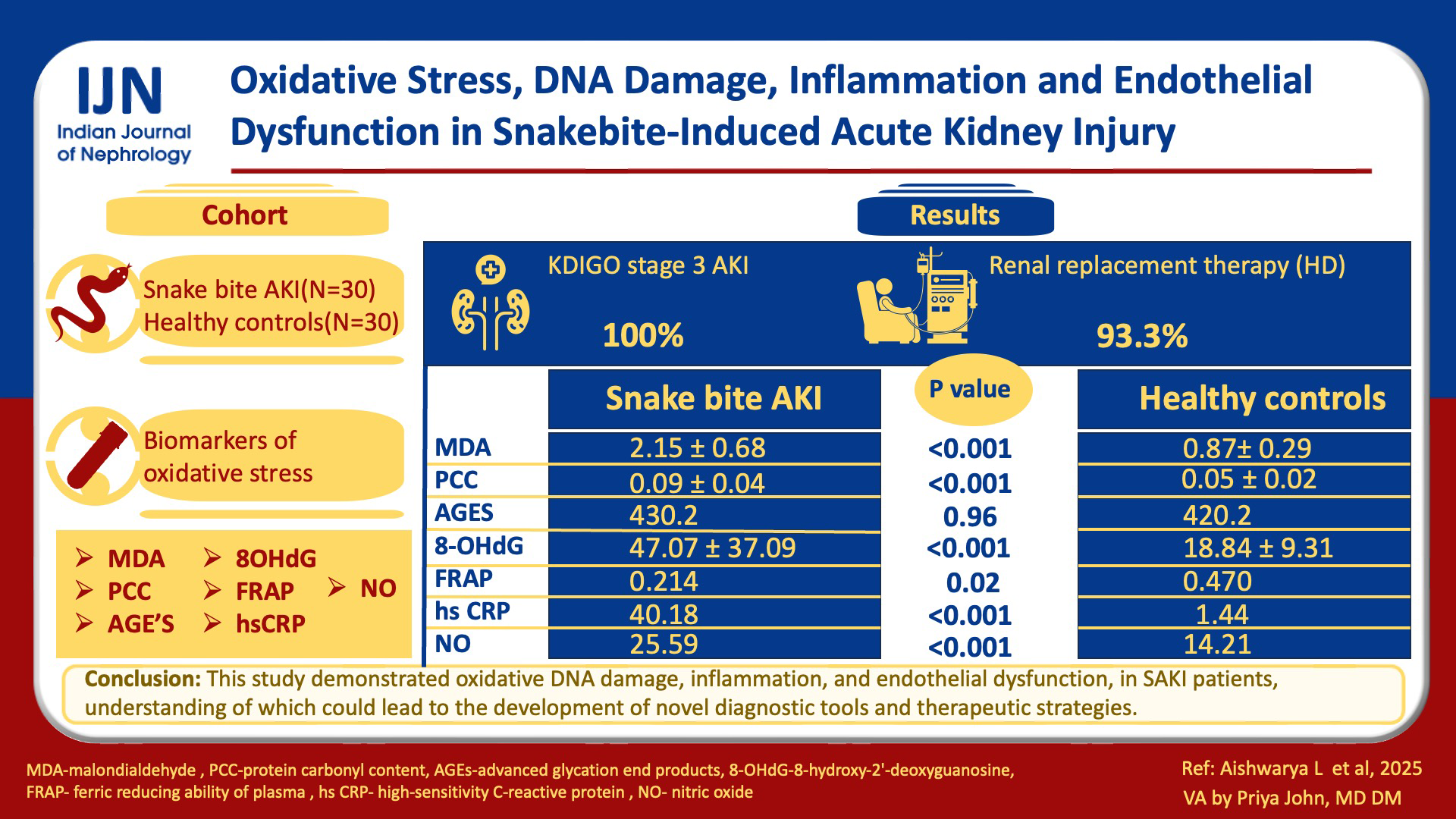
We found significantly elevated levels of MDA (2.1590±0.68221 µmol/L vs 0.8769±0.2958 µmol/L, p = <0.001), PCC (0.0905±0.040 nmol/L vs 0.0501±0.024 nmol/L, p = <0.001) and 8-OHdG (47.0757±37.09105 ng/mL vs 18.8450±9.31479 ng/mL, p = <0.001) in SAKI patients compared to controls, indicating increased oxidative damage to lipids, proteins, and DNA respectively. Although AGEs showed higher levels in SAKI patients, the difference was not significant. FRAP levels were significantly reduced [0.214 (0.051-0.489) mmol/L vs 0.470 (0.136-0.564) mmol/L, p = 0.024], indicating compromised antioxidant capacity. Significantly elevated levels of hs-CRP [40.18 (16.96-77.56) mg/L vs 1.44 (0.5-4.45) mg/L, p = <0.001] and NO [25.59 (22.75-28.43) µmol/L vs 14.218 (11.37-16.35) µmol/L, p = <0.001] confirmed the presence of inflammation and endothelial dysfunction in these patients.
Conclusion
Our study demonstrated oxidative stress, including oxidative DNA damage, inflammation, and endothelial dysfunction, in SAKI patients. Understanding these intricate mechanisms could lead to the development of novel diagnostic tools and therapeutic strategies.
Keywords
8-OHdG
DNA damage
Endothelial dysfunction
Inflammation
Oxidative stress
SAKI
Introduction
Snakebite envenomation is potentially life-threatening and is often encountered in rural areas of tropical countries. The World Health Organization recognized it as a priority neglected tropical disease.1 Around 5.4 million snakebites occur annually worldwide, resulting in about 2.5 million envenomations and 81,000–138,000 deaths.2 Snakebites are common in India, with an estimated 46,000–58,000 deaths annually.3,4 The four most common venomous snakes (the “big four” species) with frequent human contact in India are cobra (Naja naja), krait (Bungarus caeruleus), Russell’s viper (Daboia russelii), and saw-scaled viper (Echis carinatus).5 Acute kidney injury (AKI) is a life-threatening systemic effect of snake envenomation, most common with bites from the Viperidae and Elapidae families. In India, Russell’s viper and Echis carinatus are responsible for the majority of snakebites, and the incidence of AKI following bites from these snakes is between 13% and 32%.6,7
The mortality rate of snakebite-induced acute kidney injury (SAKI) remains high despite advances in management, emphasizing the necessity for novel therapeutic interventions to enhance patient outcomes. Recent advancements have provided valuable insights into the involvement of oxidative stress, inflammation, and endothelial dysfunction in the development of SAKI.8–11 However, the existing literature on this topic is still limited. This study investigated the presence of oxidative stress, including oxidative DNA damage, inflammation, and endothelial dysfunction in patients with SAKI. We evaluated multiple biomarkers, including malondialdehyde (MDA), protein carbonyl content (PCC), advanced glycation end products (AGEs), 8-hydroxy-2’-deoxyguanosine (8-OHdG), and ferric reducing ability of plasma (FRAP) to assess the extent of oxidative damage to lipids, proteins, and DNA as well as overall antioxidant capacity. We measured high-sensitivity C-reactive protein (hs-CRP) as an indicator of inflammation and nitric oxide (NO) as a marker of endothelial dysfunction.
Materials and Methods
This cross-sectional, observational study was conducted at Sri Venkateswara Institute of Medical Sciences, Tirupati, a tertiary care teaching hospital in South India, after obtaining approval from the Institutional Ethics Committee (approval number: 1070). Thirty consecutive patients with SAKI, admitted to the Nephrology department between December 2020 and December 2021, were included in the study. Additionally, 30 healthy individuals were included as control subjects. Informed consent was obtained from all individual participants included in the study. The diagnosis of snakebite was established based on the presence of one or more of the criteria, including the presence of fang marks, signs of envenomation, accounts from witnesses or patients who saw the snake responsible for the bite, and a history of anti-snake venom (ASV) administration. Patients with chronic kidney disease (CKD) and AKI due to other causes were not included in the study. The clinical history was noted and a physical examination was done. A 20-minute whole blood clotting test and coagulation profile (Prothrombin time, international normalized ratio, and activated partial thromboplastin time) were assessed. Polyvalent ASV was administered as needed. AKI diagnosis and staging were done by Kidney Disease Improving Global Outcomes (KDIGO) criteria.12 Acute Physiology Age and Chronic Health Evaluation II (APACHE II), Sequential Organ Failure Assessment (SOFA), and Acute Tubular Necrosis-Individual Severity Index (ATN-ISI) scores were determined.13–15
For biochemical analysis, 6 mL of venous sample was collected from SAKI patients at the time of admission to the intensive care unit. From that, 4 mL was collected into additive-free tubes and 2 mL of the sample was transferred to heparinized vials. The blood samples were centrifuged at 3000 rpm for 15 minutes and the separated serum and plasma were stored at ‒800C until further analysis. Serum creatinine and urea were estimated using commercial kits; hs-CRP was estimated by the immunoturbidimetry method using Beckman system pack. All the above parameters were analyzed on clinical chemistry AU 480, Beckman coulter auto analyzer (California, USA). Plasma MDA, FRAP, and PCC were estimated using spectrophotometric methods on PerkinElmer Lambda 25 UV-VIS Spectrophotometer, U.S.A.16-18 Serum nitric oxide was estimated by Griess Method.19 AGEs and 8-OHdG were estimated by enzyme-linked immunosorbent assay (ELISA) with ELISA reader (Transasia Bio-Medicals Ltd, Mumbai, India) and ELISA washer (ERBA Diagnostics, Mannheim, Germany) and using commercial kits (Genxbio Health Sciences Pvt Ltd, Greater Noida, India).
Statistical analysis
The normal distribution of all continuous variables was assessed using the Shapiro-Wilk test. Normally, distributed values were presented as mean ± standard deviation, while non-normally distributed values were presented as median (interquartile range). Categorical variables were reported as numbers and percentages. For comparisons between two groups with continuous variables, the student’s unpaired t-test or Mann-Whitney test was used, depending on the distribution of the data. χ2-test was used in the analysis of categorical variables. Pearson’s rank correlation or Spearman correlation was employed to determine correlations between variables. Statistical analyses were performed using SPSS (version 16.0, SPSS Inc., Chicago, IL, USA). A p <0.05 was considered significant.
Results
The study group consisted of 30 patients who experienced AKI because of snakebite, while 30 healthy individuals were included as controls. To assess oxidative stress, several biomarkers were employed. These included MDA, a marker of lipid peroxidation and oxidative damage to cell membranes. PCC was measured as an indicator of protein oxidation, while AGEs which are modifications of proteins or lipids that become nonenzymatically glycated and oxidized after contact with reducing sugars were examined as markers of oxidative stress-related protein modifications. The 8-OHdG served as a marker for oxidative DNA damage. The FRAP assay was utilized to evaluate the overall antioxidant capacity. Inflammation was assessed using hs-CRP, a well-known marker of systemic inflammation. Endothelial dysfunction, which refers to impaired function of the blood vessel lining, was evaluated using NO as a biomarker.
The mean age of the SAKI group was 46.27 ± 11.82 years, ranging from 18 to 64 years, and the control group had a mean age of 45.07 ± 9.70 years, ranging from 20 to 58 years. The mean serum creatinine of the control group was 0.87 ± 0.21 mg/dl. In both these groups, males were predominant. The demographic and clinical characteristics of patients with SAKI were tabulated [Table 1]. Most patients (96.6%) received polyvalent ASV. All patients were in KDIGO stage 3 AKI, and 93.3% intermittent hemodialysis or sustained low-efficiency dialysis. Three patients (10%) died. The laboratory results and the frequency of different biochemical and hematological manifestations observed in SAKI patients were tabulated [Table 2]. Patients who were oligo-anuric or did not experience a satisfactory decrease in serum creatinine levels after two weeks underwent kidney biopsy. The predominant lesion was acute tubular necrosis with intratubular pigmented granular casts (57.14%), followed by acute interstitial nephritis (28.57%) and a combination of acute tubular injury and acute interstitial nephritis (14.28%).
| Age (years) | 46.26667 ± 11.82322 |
| Gender | |
| Male | 22 (73.3%) |
| Female | 8 (26.6%) |
| Place | |
| Rural | 24 (80%) |
| Urban | 6 (20%) |
| Bite site | |
| Lower limb | 24 (80%) |
| Upper limb | 6 (20%) |
| Coagulation defect | 26 (86.6%) |
| Neurotoxicity | 11 (36.6%) |
| Local features (limb swelling/cellulitis) | 29 (96.6%) |
| Oliguria/Anuria | 25 (83.3%) |
| Hematuria | 23 (76.6%) |
| ASV given | 29 (96.6%) |
| Number of ASV vials | 20.07 ± 10.075 |
| Bite to ASV time in hours (Median [IQR]) | 5 (4–9) |
| Bite to HD time in hours (Median [IQR]) | 48 (27–32) |
| Hypotension | 6 (20%) |
| Mechanical ventilation requirement | 4 (13.3%) |
| KDIGO stage 3 AKI | 30 (100%) |
| Renal replacement therapy (HD) | 28 (93.3%) |
| Number of HD sessions | 5.73 ± 3.75 |
| Requirement of plasmapheresis | 3 (10%) |
| APACHE II score | 16.23 ± 3.848 |
| SOFA score | 6.70 ± 2.996 |
| ATN-ISI score | 0.55513 ± 0.134537 |
| Duration of hospital stay (days) | 12.47 ± 6.107 |
| Mortality | 3 (10%) |
IQR: Inter quartile range, ASV: Anti-snake venom, HD: hemodialysis, KDIGO: Kidney disease improving global outcomes, AKI: Acute kidney injury, APACHE II: Acute physiology age and chronic health evaluation II, SOFA: Sequential organ failure assessment, ATN-ISI: Acute tubular necrosis-individual severity index.
| Laboratory results of snake-bite-induced acute kidney injury patients | |
|---|---|
| Parameter | Value |
| S. Creatinine (mg/dL) | 6.92 ± 4.27α |
| Hemoglobin (gm/dL) | 10.90 ± 2.69α |
| Platelet count (lakhs/cu mm) | 1.33 ± 0.795α |
| Total leucocyte count (cells/cu mm) | 15,336.67 ± 6891.59α |
| S. Total bilirubin (mg/dL) | 1.35 (0.725–3.9)β |
| S. Direct bilirubin (mg/dL) | 0.47 ± 0.41α |
| AST (IU/L) | 79.6 (35-12-132.25)β |
| ALT (IU/L) | 32 (22.25–46.25)β |
| S. Albumin (gm/dL) | 3.22 ± 0.39α |
| S. Lactate (mg/dL) | 8 (5.8–12)β |
| S. Procalcitonin (ng/mL) | 0.515 (0.24–6.8)β |
| S. LDH(IU/L) | 700 (480–911)β |
| S. CPK(U/L) | 446 (205.25–1372)β |
| S. Uric acid (mg/dL) | 7.06 ± 1.63α |
| Frequency of various biochemical and hematological manifestations in snake-bite-induced acute kidney injury patients (n = 30) | |
| Parameter | n (%) |
| Anemia (Hemoglobin <10 gm/dL) | 14 (46.6%) |
| Thrombocytopenia (Platelets <100,000/mm3) | 11 (36.6%) |
| Leukocytosis | 16 (53.3%) |
| Coagulopathy | 26 (86.6%) |
| Hypoalbuminemia (serum albumin <3.5 gm/dL) | 22 (73.3%) |
| Hyperbirubinemia (Total bilirubin >1.2 gm/dL) | 16 (53.33%) |
| Hyperkalemia | 8 (26.6%) |
| Hemoglobinuria | 17 (56.6%) |
| Myoglobinuria | 23 (76.6%) |
| Features of thrombotic microangiopathy | 3 (10%) |
AST: Aspartate transaminase, ALT: Alanine transaminase, LDH: Lactate dehydrogenase, CPK: creatine phosphokinase: α: mean ± standard deviation, β: Median (inter quartile range).
A comparison of biomarkers of oxidative stress, including oxidative DNA damage, inflammation, and endothelial dysfunction between the study groups, is shown in Table 3. MDA was significantly high in the SAKI group compared to controls (p < 0.001). Similarly, the level of PCC in the SAKI group was significantly higher than in the control group (p < 0.001). Though the level of AGEs in the SAKI group was slightly higher than in the control group, the difference was not significant (p = 0.962). SAKI group exhibited a significantly elevated level of 8-OHdG compared to the control group (p < 0.001).
| Parameter | Snake-bite AKI patients (n = 30) | Healthy controls (n = 30) | p-value |
|---|---|---|---|
| MDA (µmol/L) | 2.1590 ± 0.68221α | 0.8769 ± 0.29586α | <0.001* |
| PCC (nmol/L) | 0.0905 ± 0.040α | 0.0501 ± 0.024α | <0.001* |
| AGEs (ng/L) | 430.2 (338.76-551.45)β | 420.2 (328.82-1093.37)β | 0.962 |
| 8-OHDG (ng/mL) | 47.0757 ± 37.09105α | 18.8450 ± 9.31479α | <0.001* |
| FRAP (mmol/L) | 0.214 (0.051-0.489)β | 0.470 (0.136-0.564)β | 0.024* |
| hs-CRP (mg/L) | 40.18 (16.96-77.56)β | 1.44 (0.5-4.45)β | <0.001* |
| NO (µmol/L) | 25.59 (22.75-28.43)β | 14.218 (11.37-16.35)β | <0.001* |
DNA- Deoxyribonucleic acid, AKI: Acute kidney injury, MDA: Malondialdehyde, PCC: protein carbonyl content, AGEs: Advanced glycation end products, 8-OHDG: 8-hydroxy-2’-deoxyguanosine, FRAP: Ferric reducing ability of plasma, hs-CRP: High sensitivity C-reactive protein, NO: Nitric oxide, α: mean ± standard deviation, β: Median (inter quartile range), *: Significant.
The level of FRAP in the SAKI group was significantly lower than the control group’s level (p = 0.024). The inflammatory stress marker hs-CRP in the SAKI group was significantly high compared to the control group (P < 0.001). The SAKI group exhibited significantly higher levels of NO compared to the control group (p < 0.001).
The AKI prognosis scoring systems (APACHE II, SOFA, and ATN-ISI) exhibited a positive correlation with biomarkers MDA, PCC, AGEs, 8-OHDG, hs-CRP, and NO. Conversely, a negative correlation was noted with the antioxidant capacity marker FRAP, suggesting increased levels of oxidative stress, DNA damage, inflammation, and endothelial dysfunction with higher scores on these prognosis scoring systems. However, significant positive correlations were only observed between the APACHE II score and levels of AGEs and FRAP [Table 4].
| Variables | MDA | PCC | AGEs | 8-OHdG | FRAP | NO | HS CRP | |
|---|---|---|---|---|---|---|---|---|
| MDA | Correlation coefficient | 1 | 0.106 | 0.451 | 0.244 | ‒0.188 | 0.163 | 0.339 |
| P-value | <0.00001 | 0.577 | 0.012* | 0.193 | 0.319 | 0.389 | 0.066 | |
| PCC | Correlation coefficient | 0.106 | 1 | 0.236 | 0.046 | ‒0.221 | 0.036 | 0.159 |
| P-value | 0.577 | <0.00001 | 0.209 | 0.809 | 0.240 | 0.85 | 0.401 | |
| AGEs | Correlation coefficient | 0.451 | 0.236 | 1 | 0.222 | ‒0.007 | 0.165 | 0.051 |
| P.value | 0.012* | 0.209 | <0.00001 | 0.208 | 0.970 | 0.383 | 0.788 | |
| 8-OHdG | Correlation coefficient | 0.244 | 0.046 | 0.222 | 1 | ‒0.247 | 0.205 | 0.138 |
| P-value | 0.193 | 0.809 | 0.238 | <0.00001 | 0.188 | 0.277 | 0.467 | |
| FRAP | Correlation coefficient | ‒0.188 | ‒0.221 | ‒0.007 | ‒0.247 | 1 | ‒0.239 | ‒0.140 |
| P-value | 0.319 | 0.240 | 0.970 | 0.188 | <0.00001 | 0.203 | 0.460 | |
| NO | Correlation coefficient | 0.163 | 0.036 | 0.165 | 0.205 | ‒0.239 | 1 | 0.041 |
| P-value | 0.389 | 0.856 | 0.383 | 0.277 | 0.203 | <0.00001 | 0.829 | |
| HS CRP | Correlation coefficient | 0.339 | 0.159 | 0.051 | 0.138 | ‒0.140 | 0.041 | 1 |
| P-value | 0.066 | 0.401 | 0.788 | 0.467 | 0.460 | 0.829 | <0.00001 | |
| APACHE II score | Correlation coefficient | 0.059 | 0.230 | 0.363 | 0.076 | ‒0.410 | 0.184 | 0.077 |
| P-value | 0.756 | 0.221 | 0.048* | 0.689 | 0.024* | 0.330 | 0.685 | |
| SOFA score | Correlation coefficient | 0.072 | 0.284 | 0.199 | 0.011 | ‒0.134 | 0.208 | 0.166 |
| P-value | 0.705 | 0.1282 | 0.291 | 0.953 | 0.480 | 0.270 | 0.380 | |
| ATN-ISI (Liano score) | Correlation coefficient | 0.115 | 0.330 | 0.282 | 0.077 | ‒0.221 | 0.255 | 0.009 |
| P-value | 0.546 | 0.074 | 0.131 | 0.685 | 0.240 | 0.173 | 0.962 |
DNA: Deoxyribonucleic acid, AKI: Acute kidney injury, MDA: Malondialdehyde, PCC-protein carbonyl content, AGEs: Advanced glycation end products, 8-OHDG: 8-hydroxy-2’-deoxyguanosine, FRAP: Ferric reducing ability of plasma, hs-CRP: High sensitivity C-reactive protein, NO: Nitric oxide, APACHE II: Acute physiology age and chronic health evaluation II, SOFA: Sequential organ failure assessment, ATN-ISI: Acute tubular necrosis-individual severity index, *: Significant.
The biomarkers, MDA, PCC, AGEs, 8-OHDG, NO, and hs-CRP, exhibited a positive correlation, indicating that higher levels of one tend to coincide with elevated levels of the others. A negative correlation was observed between these biomarkers and FRAP, indicating a decrease in antioxidant capacity as these biomarker levels increase. However, a significant positive correlation was only observed between MDA and AGEs [Table 4].
Median levels of all biomarkers, except FRAP, were higher in the non-survivor group compared to the survivor group of SAKI, although this elevation did not reach statistical significance [Table 5].
| Parameter |
Survivors (n = 27) Median (IQR) |
Nonsurvivors (n = 3) Median (IQR) |
P Value |
|---|---|---|---|
| MDA (µmol/L) |
2.136 (1.505-2.502) |
2.207 (1.941-2.625) |
0.744 |
| PCC (nmol/L) |
0.091 (0.058-0.112) |
0.125 (0.090-0.141) |
0.349 |
| AGEs (ng/L) |
404.7 (335.800-528.425) |
507.800 (491.60-2566.400) |
0.197 |
| 8-OHDG (ng/mL) |
15.52 (15.245-27.200) |
37.09 (31.895-53.395) |
0.226 |
| SFRAP (mmol/L) |
0.493 (0.275-0.517) |
0.088 (0.051-0.477) |
0.422 |
| hs-CRP (mg/L) |
8.510 (5.220-27.920) |
44.620 (18.850-79.720) |
0.117 |
| NO (µmol/L) |
25.592 (22.749-29.858) |
28.436 (25.592-28.436) |
0.626 |
AKI: Acute kidney injury, IQR: Inter quartile range, MDA: Malondialdehyde, PCC: protein carbonyl content, AGEs: Advanced glycation end products, 8-OHDG: 8-hydroxy-2’-deoxyguanosine, FRAP: Ferric reducing ability of plasma, hs-CRP: High sensitivity C-reactive protein, NO: Nitric oxide
Discussion
The pathogenesis of SAKI likely involves various factors such as direct nephrotoxic effects of venom, coagulopathy, hypotension, intravascular haemolysis, and disseminated intravascular coagulation. Complications like capillary leak syndrome and thrombotic microangiopathy contribute to endothelial dysfunction and renal tissue damage. Complement activation, sepsis, and hypersensitivity reactions to venom or antivenom proteins play significant roles in the development of SAKI.6,7 Amidst this complexity, oxidative stress emerges as a critical mediator.8–11 This multifaceted interplay underscores the importance of addressing oxidative stress alongside other pathogenic mechanisms in the comprehensive management of SAKI.
Oxidative stress arises when there’s a discrepancy between the generation of free radicals and the capacity of cells to eliminate them efficiently.20 Snake venom contains phospholipases A2, metalloproteinases, hyaluronidases, L-amino acid oxidase, three-finger toxins, and various other toxins that can lead to the production of ROS and disrupt the balance between oxidative stress and antioxidant defences.8
ROS disrupts endothelial cell function, impairing blood flow regulation and promoting renal ischemia. Venom toxins target mitochondria, producing excess ROS, further amplifying oxidative stress, especially in proximal tubular cells reliant on oxidative phosphorylation for energy. DNA damage from free radicals can cause cellular dysfunction, cell cycle arrest, and cell death. AKI-associated cell cycle arrest increases cytokine secretion and fibrosis development, driving progression to CKD. Antioxidant systems within the body help neutralize ROS and minimize oxidative damage.20–22 ROS are tracked by measuring stable molecular products resulting from their reaction with biomolecules such as DNA, proteins, and lipids.23
In our study, a significantly elevated MDA, an end-product of lipid peroxidation, was observed in the SAKI group compared to the control group. Lipid peroxidation can damage cells by impairing the plasma membrane and subcellular organelles, increasing ROS levels and causing damage to cellular proteins and DNA, and activating phospholipase A2 which damages the cell membrane.24 Two other studies also reported significantly increased levels of thiobarbituric acid reactive substances which are equivalent to MDA in SAKI patients.9,10
PCC is an indicator of oxidative protein modification, which results in the alteration of protein structure, function, and stability leading to cellular dysfunction and damage.25 Significantly elevated levels of PCC were noted in SAKI patients compared to controls in our study. These findings align with three other studies,9–11 which also reported significantly heightened levels of advanced oxidation protein products that are also a marker of oxidative protein damage in SAKI patients.
AGEs, formed via nonenzymatic glycation when reducing sugars interact with proteins, lipids, or nucleic acids, progress from labile Schiff’s base to stable Amadori products, and ultimately to stable and irreversible AGEs over weeks or months through oxidative decomposition and molecular rearrangements.26 Although the SAKI group exhibited slightly elevated AGEs levels compared to controls, the difference lacked significance, likely due to the gradual formation process. Methylglyoxal, an AGEs precursor, showed significant elevation in SAKI patients in two studies,9,11 while one study reported significantly increased AGEs levels in SAKI patients.9
8-OHdG, an oxidized form of guanine, is a marker of oxidative DNA damage.27 The scarcity of existing studies investigating 8-OHdG levels in SAKI highlights a gap in our understanding of the potential involvement of oxidative DNA damage in this context. In this study, serum levels of 8-OHdG were significantly higher in the SAKI group compared to controls, reflecting the extent of DNA damage.
FRAP assay is a method for assessing the antioxidant power.17 The SAKI patients exhibited significantly lower levels of FRAP, indicating reduced antioxidants, compared to the control group. Two other studies also demonstrated significantly reduced total antioxidant status in SAKI patients.9,11 These observations underscore the importance of antioxidant defense mechanisms in mitigating oxidative stress and highlight potential targets for therapeutic interventions in the management of SAKI.
The hs-CRP, a commonly used marker of systemic inflammation,28 was elevated in the SAKI group. Although there are currently no other existing studies specifically investigating hs-CRP levels in SAKI, our findings suggests a pronounced inflammatory response in these patients.
NO, a potent vasodilator crucial for endothelial function, was significantly elevated in SAKI patients compared to controls in our study. While endothelial dysfunction is often associated with reduced NO production, endothelial activation often precedes overt endothelial dysfunction, which explains the elevated levels of NO in the SAKI group. Inflammatory mediators can stimulate the production of inducible nitric oxide synthase, leading to an increased generation of NO. While initially beneficial, sustained elevation of NO can lead to detrimental effects due to the formation of reactive nitrogen species, causing oxidative stress and endothelial damage.29
A pattern of heightened oxidative stress, inflammation, and endothelial dysfunction was evident with increasing scores of AKI prognosis scoring systems and a trend of mutual elevation in biomarkers was observed, significant correlations were only identified between the APACHE II score and levels of AGEs and FRAP as well as between MDA and AGEs.
Oxidative injury represents a common pathological mechanism implicated in the pathogenesis of AKI across various etiologies, extending beyond the specific context of SAKI.30 Recognizing the shared mechanisms underlying renal injury facilitates the development of broad-spectrum therapeutic strategies aimed at mitigating oxidative injury and improving patient outcomes.
Our study thus demonstrated early and widespread cellular- and molecular-level injury through immediate oxidative stress, inflammation, endothelial dysfunction, and plausible distant effects through DNA damage in SAKI patients, reinforcing the awareness to implement the preventive and therapeutic strategies at the earliest to mitigate the burden of morbidity and mortality in SAKI.
Conflict of interest
There are no conflicts of interest.
References
- Snake-bite envenoming: A priority neglected tropical disease. Lancet. 2017;390:2.
- [CrossRef] [PubMed] [Google Scholar]
- World Health Organization. Snakebite Envenoming. Available at https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming. [Accessed on February 23, 2020].
- Snakebite mortality in India: A nationally representative mortality survey. PLoS Negl Trop Dis. 2011;5:e1018.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Trends in snakebite deaths in India from 2000 to 2019 in a nationally representative mortality study. Elife. 2020;9:e54076.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diversity and distribution of medically important snakes of India. Clinical Toxinology in Asia Pacific and Africa 2015:115-36. DOI 10.1007/978-94-007-6288-6_16-1
- [Google Scholar]
- Snake-bite-induced acute renal failure in India. Kidney Int. 1989;35:891-907.
- [CrossRef] [PubMed] [Google Scholar]
- Snake envenoming─An underreported cause of acute kidney injury. Kidney Int Rep. 2019;4:643-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Inflammation and oxidative stress in snakebite envenomation: A brief descriptive review and clinical implications. Toxins (Basel). 2022;14:802.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Snakebite mediated acute kidney injury, prognostic predictors, oxidative and carbonyl stress: A prospective study. Indian J Nephrol. 2016;26:427-33.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Methylglyoxal increase in uremia with special reference to snakebite mediated acute renal failure. Clin Chim Acta. 2008;391:13-7.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma level of protein modification and inflammatory markers in snakebite induced acute kidney injury patients undergoing haemodialysis—An observational study. Indian J. Basic Appl Med Res. 2018;7:342-9.
- [Google Scholar]
- Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1-38.
- [Google Scholar]
- APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818-29.
- [PubMed] [Google Scholar]
- The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22:707-10.
- [CrossRef] [PubMed] [Google Scholar]
- Prognosis of acute tubular necrosis: An extended prospectively contrasted study. Nephron. 1993;63:21-31.
- [CrossRef] [PubMed] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-8.
- [CrossRef] [PubMed] [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70-6.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464-78.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36:1440-3.
- [PubMed] [Google Scholar]
- Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal. 2016;25:119-46.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dommages créés aux biomolécules (lipides, protéines, ADN) par le stress oxydant [Oxidative stress and damages to biomolecules (lipids, proteins, DNA)] Ann Pharm Fr. 2006;64:383-9.
- [CrossRef] [PubMed] [Google Scholar]
- Induction of oxidative stress in kidney. Int J Nephrol. 2012;2012:465897.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Oxidative stress tests: Overview on reliability and use. Part I. Eur Rev Med Pharmacol Sci. 2007;11:309-42.
- [Google Scholar]
- Lipid peroxidation contributes to hydrogen peroxide induced cytotoxicity in renal epithelial cells. Kidney Int. 1996;49:88-93.
- [CrossRef] [PubMed] [Google Scholar]
- Protein carbonylation. Antioxid Redox Signal. 2010;12:323-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Advanced glycation end products and the kidney. Am J Physiol Renal Physiol. 2005;289:F645-59.
- [CrossRef] [PubMed] [Google Scholar]
- 8-hydroxy-2’ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120-39.
- [CrossRef] [PubMed] [Google Scholar]
- High-sensitivity C-reactive protein: Clinical importance. Curr Probl Cardiol. 2004;29:439-93.
- [PubMed] [Google Scholar]
- Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002;61:855-61.
- [CrossRef] [PubMed] [Google Scholar]
- Acute renal failure in falciparum malaria: Clinical characteristics, demonstration of oxidative stress, and prognostication. Saudi J Kidney Dis Transpl. 2012;23:296-300.
- [PubMed] [Google Scholar]








