Translate this page into:
Pattern of biopsy-proven renal disease in a single center of south India: 19 years experience
Address for correspondence: Dr. Uttara Das, Department of Nephrology, Nizam's Institute of Medical Sciences, Punjagutta, Hyderabad - 500 082, India. E-mail: druttaradas@rediffmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The prevalence of biopsy-proven glomerulonephritis varies according to the geographic area, socioeconomic condition, race, age, demography and indication of renal biopsy. This study analyzed the distribution of biopsy-proven renal disease (BPRD) and its changing pattern over a period of 19 years from a tertiary care hospital in south India. All the renal biopsies performed from 1990 to 2008 were reviewed retrospectively. Biopsies were evaluated by light microscopy and immunofluorescence microscopy and also special stains when warranted. A total of 1849 biopsies were analyzed. The mean patient age was 32.27 ± 18.38 (range 10-80) years. The male:female ratio was 1.4:1. The most common indications of renal biopsy were nephrotic syndrome (49%), followed by chronic renal failure (13.6%) and rapidly progressive renal failure (12%). Primary glomerulonephritis (PGN) comprised 1278 (69.1%) of the total patients. Among the PGN cases, the most common one was minimal change disease (21.8%), followed by focal segmental glomerulosclerosis [FSGS (15.3%)], membranous glomerulonephritis (10%), chronic glomerulonephritis (9.7%), postinfectious glomerulonephritis (8.1%), mesengioproliferative glomerulonephritis (7.5%), diffuse proliferative glomerulonephritis (6.7%), crescentic glomerulonephritis (6.5%), IgA nephropathy [IgAN (6.3%)], membranoproliferative glomerulonephritis (5.7%), focal proliferative glomerulonephritis (1.6%) and IgM nephropathy (0.5). Secondary glomerular disease (SGN) accounted for 337 (18.2%) of the cases. The most common SGN was lupus nephritis (80.1%), followed by amyloidosis (8%) and diabetic nephropathy (6.5%). Tubulointerstitial disease [124 (6.7%)] and vascular disease [60 (3.2%)] were less common. End-stage changes and miscellaneous disease were found in 37 (2%) and 13 (0.7%) cases, respectively. The incidence of FSGS and IgAN has been increasing since 1999. This study provides descriptive biopsy data and highlights the changing incidence of renal disease which is probably contributed by an increase referral due to increased awareness together with increased manpower and infrastructure.
Keywords
Epidemiology
glomerulonephritis
nephropathy
renal biopsy
Introduction
Renal biopsy is a definitive diagnostic test in patients with renal parenchymal disease. Indications of renal biopsy vary from center to center.[1] Renal biopsy is useful for identifying the specific diagnosis, assessing the level of disease activity, and for allowing specific decisions about treatment to be made. The common clinical situations where biopsy is needed are nephrotic syndrome (NS), prolonged acute renal failure (ARF), rapidly progressive renal failure (RPRF), systemic diseases with renal dysfunction, non-nephrotic proteinuria, isolated microscopic hematuria, unexplained chronic renal failure (CRF), renal transplant dysfunction, and familial renal diseases.[2]
Renal biopsy data analysis is essential to study the prevalence of biopsy-proven renal disease (BPRD) and its variation and distribution as per geographic areas, socioeconomic conditions, race, age and indication for renal biopsy, to understand the regional epidemiology of glomerular disease in a particular geographical region. It also improves the understanding of the utility of renal biopsy and acts as a framework for future research into renal parenchymal disease. Unfortunately, we do not have a central biopsy registry in India. Studies on the prevalence of renal disease in India are limited.[3–6] Evidence from different published articles across the world indicates a changing pattern of glomerular disease over the last few decades.[7–21] We have completed 19 years of collection of renal biopsy data at our center, a referral tertiary care teaching hospital in south India, and report the pattern of BPRD.
Materials and Methods
All the kidney biopsies performed in our institute from January 1990 to December 2008 were retrospectively analyzed. We recorded the following data for each patient: name, age, sex, indication for renal biopsy, histopathological diagnosis and laboratory investigations such as serum creatinine, 24-hour urinary protein, urine microscopy, virology (HBsAg, anti-HCV, HIV) and serology [anti-dsDNA antibody, antinuclear antibody (ANA), C3, C4]. All renal biopsy specimens obtained were prepared as per the standard protocol and examined by the same group of pathologists and technicians of our institute. Analysis included light microscopy (LM) and immunofluorescence (IF). However, electron microscopy (EM) was not systematically performed as this facility is not available. For LM, three sections were stained with Hematoxylin and Eosin, one with periodic acid Schiff, one with Masson's trichrome, and one with Jones silver methanamine. Special stains were used when warranted. IF study was done by using polyclonal antisera (FITC-conjugated Rabbit Antihuman Antisera manufactured by DAKO from Denmark) against human IgG, IgM, IgA, C3, C1q, and kappa and lambda light chains. During the period from 1990 to 1997, IF analysis was performed only in a few biopsies. From 1998 to 2008, IF was done in all biopsies. The indications for renal biopsy were categorized into seven clinical syndromes: NS, acute nephritic syndrome (ANS), asymptomatic urinary abnormalities (AUA), hematuria, ARF, CRF, and RPRF. Standard definitions of the clinical syndrome were used.[2223] In CRF, renal biopsy was performed for unexplained renal failure if kidney sizes were within normal limit with intact corticomedullary differentiation. A tru cut biopsy needle was used for all the biopsies done from 1990 to 2000, and whereas automated biopsy guns were used from 2001 to 2008. Histological categories were classified as follows: I) primary glomerulonephritis (PGN) which included minimal change disease (MCD), FSGS, membranous nephropathy (MN), IgA nephropathy (IgAN), IgM nephropathy (IgMN), mesangioproliferative glomerulonephritis (MesPGN), membranoproliferative glomerulonephritis (MPGN), crescentic glomerulonephritis (CresGN), diffuse proliferative glomerulonephritis (DPGN), postinfectious glomerulonephritis (PIGN); II) secondary glomerulonephritis (SGN) included lupus nephritis (LN), diabetic nephropathy (DN), amyloidosis (AM), Henoch–Schönlein purpura (HSP), multiple myeloma (MM), light chain deposit disease (LCDD), systemic vasculitis (VAS), hemolytic–uremic syndrome (HUS)/ thrombotic microangiopathy (TTP), systemic sclerosis; III) tubulointerstitial nephritis (TIN) included acute TIN, chronic TIN, acute tubular necrosis (ATN); IV) vascular nephropathy (VN) included benign/malignant nephrosclerosis, thrombotic microangiopathy (TMA), acute cortical necrosis, hypertensive changes; V) hereditary; VI) end-stage renal disease (ESRD) changes and VII) miscellaneous included no significant changes and histological categories whose number is less than 5. ESRD changes are characterized by advanced glomerulosclerosis, tubular loss/atrophy, some degree of cystic change and thickened renal blood vessels.[24] We calculated the incidence of each type of renal disease and indication of biopsy. Comparison was made between data from 1990 to 1998 and from 1999 to 2008. The data generated and analyzed were also compared with studies published from India and different regions of the world.
Simple descriptive statistics such as median and mean ± SD were used for variables such as age, clinical and laboratory features. Percentage was used for categorical data. Graphs were generated with Microsoft Excel 2007.
Results
A total of 2401 renal biopsies were analyzed retrospectively from 1990 to 2008, out of which 552 were excluded (renal allograft biopsies: n= 199, incomplete data or inadequate biopsies: n= 353). The remaining 1849 patients were included in the study. Among them, 1091 were males and 758 were females. The mean age of patients was 32.27 ± 18.37 (range 10-80) years. The number of patients who underwent renal biopsies had been increasing annually [Figure 1].

- Total number of renal biopsies (Bx) performed in each year from 1990-2008
The most common indication for renal biopsy was NS: 906 (49%), followed by CRF: 251 (13.6%), RPRF: 221 (12%), ANS:167 (9%), AUA:167 (9%), ARF:120 (6.5%) and gross hematuria:17 (0.9%).
The overall frequencies of different renal diseases in native renal biopsies together with some basic data for each disease are shown in Table 1. From the data collected and analyzed, it can be seen that PGN remained the most common and important kidney disease in our patients and accounted for 1278 (69.1%) of the total patients. Among the PGN cases, MCD (21.8%) was the leading category, followed by FSGS (15.3%), MN (10%), chronic glomerular nephritis CGN (9.7%), PIGN (8.1%), MesPGN (7.5%), DPGN (6.7%), CresGN (6.5%), IgAN (6.3%), MPGN (5.7%) and focal proliferative glomerular nephritis [FPGN] (1.6%). IgMN (0.5%) was very rare. The diagnosis of IgMN was made after ruling out MCD and FSGS.

The most common SGN (n = 337) was LN (80.1%), followed by amyloidosis (8%) and DN (6.5%). TIN, VN and ESRD changes were less common diagnostic categories. There were no hereditary glomerular diseases in this analysis.
Table 2 shows the clinical syndrome underlying in each histological category. It is obvious from the table that most frequent causes of NS were MCD, MN and FSGS. Though only 5-10% patients of IgAN presented as NS, in this study we observed NS in 44.4% patients. This probably reflects a selection bias. Out of 13 miscellaneous cases, 3 had LCDD and presented as NS. In the remaining nine cases, the histopathology did not show significant change and had a varied symptomatology. None of these cases had IF available.

By the parameter age of the patient, most of the PGN was diagnosed between second and fourth decades as shown in Table 3.

Figure 2 depicts the frequency of PGN in the two periods. The frequency of PGN had increased during the period 1999-2008 probably due to an increased referral as a result of increased awareness among the physicians and patients, together with increased infrastructure. Moreover, during the periods, the technique of renal biopsy had improved, making it a logically safe and an easy procedure. Hence, acceptance of the procedure by the patients was high. Increased manpower could be another reason. The incidence of FSGS and IgAN increased significantly in the second decade. Increased incidence of IgAN was due to regular use of IF. There was no major change observed in the incidence of MPGN and MesPGN.
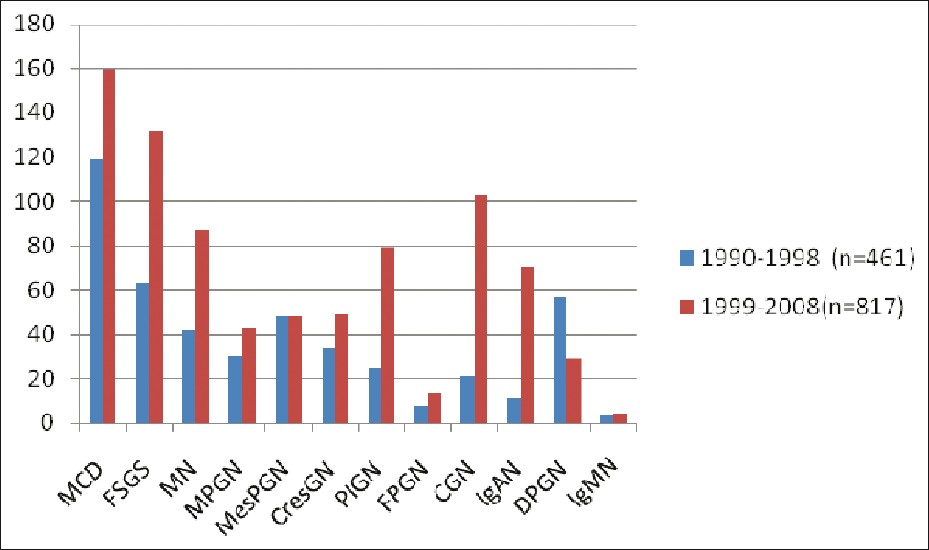
- Frequency of primary GN in two decades
LN was the most common SGN [n = 270 (80.1%)]. Male to female ratio was 1:4.6. Mean age was 28.47 ± 11 years. Most common clinical presentation was AUA (38.1%), followed by NS (37%), and RPRF (12.2%). In 12 patients, anti-dsDNA antibody was negative. HTN was present in 152 (56.3%) cases. Class IV was the most common histopathological subgroup, followed by class III, class II, class V, class VI and class I in this order. Renal insufficiency (serum creatinine > 1.5 mg/dl) was present in 88 (32.6%) cases.
Discussion
This study provides comprehensive information about the demographics, clinical syndromes and pattern of kidney diseases diagnosed by renal biopsy during a period of 19 years in a single tertiary care referral institute in south India. There are several biases regarding demographical, geographical and racial characteristics, differences in indications for renal biopsy, the analyzed clinical syndromes and variations in pathological classification. Hence, comparison with different data and drawing accurate conclusions were difficult.
A comparison of the basic data and some common diseases in our series with those of other published studies from the same region and western countries is given in Tables 4 and 5. It is obvious from these two tables that the distribution pattern of major histology of renal disease in our study did not correspond to other European and some Asian series. Most of these studies are multicentric.[9–131622]


Our data show that NS was the most frequent clinical presentation at all age groups, accounting for 49% of all cases. This is similar to that reported in many studies around the world, including India and Pakistan.[561113162526] Conversely, studies from Japan and Italy reported a higher frequency of AUA, which is quite different from ours.[912]
We also observed a male predominance in the overall cases except in SGN. This reflects the increased prevalence of LN in the female population. All recently published studies worldwide showed a similar pattern.[7–1925]
The underlying etiology of NS is variable across the world. In our study, the most common cause was MCD, followed by FSGS, MN, MesPGN, and MPGN, which is in agreement with other previous studies from our country.[3] In Korea and other northeast Asian countries like Japan, the most common cause of NS was MCD, followed by MN and IgAN.[79] Our results are consistent with the results of these studies to some extent. In contrast, in Czech registry, MN and IgAN were the most frequent diagnosis.[11] On the other hand, Serbia reported MN as the most common cause and in Brazil, FSGS was the most common cause of NS, followed by MCD and MN.[1318] Our study is not comparable with these series.
PGN was the most predominant renal disease in our study as well as in all recent studies, followed by SGN and TIN.[7–13152225] The hereditary and vascular diseases were also less frequent in almost all studies. From the data and analysis, we did not observe any hereditary GN which may be due to the unavailability of EM or to the diagnosis by other noninvasive methods.
MCD has a variable geographic distribution, being more common in some Asian than in the western countries. Several studies have shown a decline in the relative frequency of MCD.[6814] This trend has not been observed in our study. In our analysis, however, it is the commonest PGD, which is in concordance with other similar studies.[3571315] Conversely, China reported a very low incidence.[25]
The distribution pattern of FSGS was also variable. There is a worldwide increase in the incidence of FSGS despite racial variation.[2127] It was the second most common PGD found in the present study. In contrast, FSGS is the most common in some studies reported from our neighboring countries and Brazil.[461320] We too observed an increasing pattern of FSGS from 1999 onward. Reasons for this observation are unknown.
Though it is believed that MN is the most common PGD in adults, a review of different literatures reveals that most of the studies have shown MN to be the third or fourth common cause of PGD.[5791115162225] Our results also support this. However, it is still common in some regions in Asia, Europe and America.[1012–1418]
IgAN was uncommon in the present series and in other studies from this region of the world.[3–6] In contrast, it is the most common primary renal disease in European countries and some Asian countries.[7–911172225] However, we too observed a significant rise in its incidence in the last decade which can be explained by the performance of IF study in each biopsy from 1998 onward and the change of biopsy policy. In suspected cases, we do biopsy when there is any abnormality in urine analysis irrespective of the degree of proteinuria. Also, in addition to this, the number of biopsies in CRF patients is increasing when the kidneys are of normal size with intact corticomedullary distinction by ultrasonogram. Significant number of these patients turned out to be IgAN.
We have also observed that there is an increase in the incidence of PIGN and CGN from 1999 onward, which again can be explained by an early referral. In comparison to other studies, we observed a higher percentage of CresGN.
It has been observed from the study and the data interpreted thereof that MPGN is a rare disease and found primarily in the adolescent age group. Several studies from different parts of the world reported a decrease in the incidence of MPGN which was explained as due to improved hygienic environments, universal precaution and vaccinations which eventually caused a reduction in infection rate except in Romania, where MPGN is the most common PGD.[16] However, the present study does not support these observations.
IgMN was the least common entity in our study. In fact, most of the studies did not mention this as a distinct category [Tables 4 and 5]. A recently published study from Pakistan reported IgMN in 2.9% cases of PGD.[4]
The most common SGN in our study was LN which is comparable with that reported in many studies across the world.[4–8111825] Amyloidosis and DN were the next frequent causes. The data of our hospital are in line with the data from China, Czech and Serbia.[111825] The incidence of other categories of SGN was very less. However, UAE and Italy reported a high incidence of amyloidosis.[1012] Similarly, a study from Pakistan and a few previous published studies from our country had reported a high incidence of amyloidosis due to the high prevalence of tuberculosis and other infectious diseases.[3426] We had performed renal biopsy only on unsuspected cases of amyloidosis. In suspected cases of amyloidosis with an underlying etiology of tuberculosis, rheumatoid arthritis or other chronic inflammatory conditions, amyloidosis was confirmed by biopsies from other sites such as rectum, gum or abdominal fat.
TIN is found to be a relatively less frequent BPRD in many studies [Tables 4 and 5]. Our study too showed a lesser incidence of TIN. We observed a higher incidence of ATN (2%) which can be explained by aggressive performance of biopsy procedure in patients with ARF with prolonged recovery without an obvious etiology. The study has less number of patients with obstetric complications as the institute does not cater to this category.
We did not find conditions like Alport Syndrome and thin basement membrane nephropathy, which reflects the lack of EM in our center.
To conclude, from the study and data analyzed, a wide variation of major histological groups in the primary glomerular diseases has been observed. However, almost across the world, the most common secondary glomerular disease has been documented as LN. The changing incidence of BPRD is probably contributed by an increased referral due to increased awareness, together with increased manpower and infrastructure. It has been also realized that it is essential and necessary to maintain a central renal biopsy registry with an increased participation of many more nephrology centers of India to obtain accurate knowledge about the incidence, spectrum and distribution of the BPRD in our country.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Current indication for renal biopsy: A questionnaire based survey. Am J Kidney Dis. 2000;35:448-57.
- [Google Scholar]
- Renal biopsy. In: Feehally John, Floege Jurgen, Johnson Richard J, eds. Comprehensive clinical Nephrology (3rd ed). Philadelphia, PA: MOSBY ELSEVIER; 2007. p. :69-75.
- [Google Scholar]
- Pattern of renal diseases observed in native renal biopsies in adult in a single center in Pakistan. Nephrology. 2011;16:87-92.
- [Google Scholar]
- Spectrum of biopsy proven renal disease and changing trends at a tropical tertiary care centre 1990-2001. Indian J Nephrol. 2003;13:29-35.
- [Google Scholar]
- Characterization of kidney lesions in Indian adults: Towards a renal biopsy registry. Nephrol. 2006;19:205-10.
- [Google Scholar]
- Changing prevalence of glomerular diseases in Korean adults: A review of 20 years of experience. Nephrol Dial Transplant. 2009;24:2406-10.
- [Google Scholar]
- Nationwide and Long-Term Survey of Primary Glomerulonephritis in Japan as Observed in 1,850 Biopsied Cases. Nephron. 1999;82:205-13.
- [Google Scholar]
- Analysis of 490 Kidney biopsies: Data from the United Arab Emirates Renal Diseases Registry. J Nephrol. 1998;11:148-50.
- [Google Scholar]
- The Czech registry of renal biopsies.Occurrence of renal diseases in the years 1994-2000. Nephrol Dial Transplant. 2004;19:3040-9.
- [Google Scholar]
- The Italian experience of the national registry of renal biopsies. Kidney Int. 2004;66:890-4.
- [Google Scholar]
- An overview on frequency of renal biopsy diagnosis in Brazil: Clinical and pathological patterns based on 9617 native kidney biopsies. Nephrol Dial Transplant. 2010;25:490-6.
- [Google Scholar]
- The racial prevalence of glomerular lesions in nephrotic adults. Am J Kidney Dis. 1996;27:647-51.
- [Google Scholar]
- The spectrum of glomerular diseases in the Kingdom of Bahrain: An epidemiological study based on renal biopsy interpretation. Transplantat Proc. 2004;36:1792-5.
- [Google Scholar]
- Epidemiology of renal disease in Romania: A 10 year review of two regional renal biopsy databases. Nephrol Dial Transplant. 2006;21:419-24.
- [Google Scholar]
- Epidemiologic data of primary glomerular diseases in western France. Kidney Int. 2004;66:905-8.
- [Google Scholar]
- Renal biopsy registry from a single centre in Serbia: 20 years of experience. Nephrol Dial Transplant. 2009;24:877-85.
- [Google Scholar]
- McQuarrie, Bruce Mackinnon, Barbara Young.Centre variation in incidence, indication and diagnosis of adult native renal biopsy in Scotland. Nephrol Dial Transplant. 2009;24:1524-8.
- [Google Scholar]
- Changing incidence of idiopathic glomerular disease in adults. J Am SocNephrol. 1995;6:413.
- [Google Scholar]
- Frequency of renal pathology in Spain 1994-1999. Nephrol Dial Transplant. 2002;17:1594-602.
- [Google Scholar]
- Introduction to glomerular disease: Clinical presentations. In: Feehally John, Floege Jürgen, Johnson Richard J, eds. Comprehensive clinical nephrology (3rd Edition). Philadelphia, PA: MOSBY ELSEVIER; 2007. p. :193-207.
- [Google Scholar]
- Renal Disease: Classification and atlas of glomerular disease (2nd ed). New York: IkaguShoin Medical Publishers Inc; 1995. p. :1-359.
- Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int. 2004;66:920-3.
- [Google Scholar]
- Spectrum of renal diseases in Indian adults. J Assoc Physicians India. 2000;48:594-600.
- [Google Scholar]
- Increasing incidence of focal segmental glomerulosclerosis among adult nephropathies. Am J Kidney Dis. 1995;26:740-50.
- [Google Scholar]







