Translate this page into:
Pediatric Glomerular Diseases in North India–Epidemiology and Clinicopathologic Correlation
#Presently working as Consultant Pathologist at Government Medical College Srinagar, J and K.
##Presently working as Consultant Nephrologist at Government Medical College Srinagar, J and K.
Address for correspondence: Dr. Asif Sadiq Wani, Zeenat Manzil, Baghat Barzulla, P.O.Sanatnagar, Srinagar, Kashmir - 190 005, India. E-mail: drasifwani@gmail.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Glomerular diseases vary with age, and it is important to investigate the spectrum of glomerular diseases in pediatric patients to help in a more precise clinical diagnosis and optimize the management of patients. We aimed to study the clinicopathologic pattern of pediatric glomerular diseases in North India.
Methods:
This is a 5-year retrospective, single-center cohort study. The database was searched to identify all pediatric patients with glomerular diseases in their native kidney biopsies.
Results:
About 2890 native renal biopsies were studied, of which 409 were pediatric glomerular diseases. The median age was 15 years with a male preponderance. Nephrotic syndrome was the most common presentation (60.8%), followed by non-nephrotic proteinuria with hematuria (18.5%), rapidly proliferative glomerulonephritis (7%), isolated hematuria (5.3%), acute nephritic syndrome (3.4%), non-nephrotic proteinuria (1.9%), and advanced renal failure (0.7%). Minimal change disease (MCD) was the most common histological diagnosis, followed by focal segmental glomerulosclerosis (17.4%), IgA nephropathy (IgAN; 10%), membranous nephropathy (6.6%), lupus nephritis (5.9%), crescentic glomerulonephritis (2.9%), and C3 glomerulopathy (2.9%). Diffuse proliferative glomerulonephritis (DPGN) was the most common histological diagnosis in patients with hematuria and non-nephrotic as well as nephrotic range proteinuria. The most common histological diagnoses for isolated hematuria and acute nephritic syndrome were IgAN and postinfectious glomerulonephritis (PIGN), respectively.
Conclusions:
MCD and lupus nephritis are the most common pediatric primary and secondary histopathologic diagnoses, respectively. The adolescent-onset glomerular diseases have a higher frequency of IgAN, membranous nephropathy, and DPGN. PIGN is still an important differential in our pediatric patients presenting with acute nephritic syndrome.
Keywords
Epidemiology
glomerular diseases
glomerulonephritis
kidney biopsy
pediatric
Introduction
Glomerular diseases have different histologic patterns and etiologies according to the age. As the age increases, rates of primary glomerular diseases decrease and secondary glomerular diseases increase.[1] Not only there is a difference in the type of glomerular disease among different age groups, but also the same varies between races and countries due to genetic and environmental factors.[2,3] With the growing interest in pediatric glomerular diseases, a number of studies are coming up with emphasis on adolescents. Adolescent renal diseases can either begin in adolescence or may just be a continuation of childhood-onset disease. The distinction is important because renal diseases between these two groups are diverse.[1]
Over the last few decades, there has been a global increase in adult and pediatric patients with chronic kidney diseases (CKDs). The treatment and prognosis of glomerular diseases varies widely according to the specific pathology. Also, renal biopsy is comparatively difficult to perform in children than in adults. Keeping these factors in mind, it is important to investigate the composition of biopsy-proven glomerular disease in pediatric patients as detailed information on the spectrum of pediatric glomerular diseases will help in a more precise clinical diagnosis, if renal biopsies are not available, and help to optimize the management of these patients.[2] We aimed to study the clinicopathologic pattern of glomerular diseases among pediatric patients in North India.
Methods
Study design, setting, and participants
This is a 5-year retrospective, single-center cohort study (from June 2016 to June 2021) conducted at Sanjay Gandhi Post-Graduate Institute of Medical Sciences, India. The database of the hospital was searched to identify all pediatric patients with their native kidney biopsies. The common patterns/etiologies of renal diseases were included in the analysis. The pediatric patients were further categorized into two age groups–young children (0–12 years old) and adolescents (13–18 years old). The time of onset of symptoms was taken into consideration for categorization into these two groups.
Exclusion criteria: We excluded patients without histologic diagnosis (inadequate biopsies), repeat biopsies, transplant renal biopsies, those with missing demographic or clinical data, isolated tubulointerstitial kidney diseases, and those over 18 years old.
Renal biopsy evaluation
Three core biopsies were taken under ultrasound guidance, one each for light microscopy, immunofluorescence, and electron microscopy (EM). Standard processing techniques were followed in all the biopsies. Recorded immunofluorescence findings included the strength of staining for IgG, IgM, IgA, C3, C1q, kappa, and lambda, graded on a 1–4 semi-quantitative scale. Each biopsy was diagnosed according to the standard definitions and criteria using light microscopy, immunofluorescence, and EM, wherever necessary. Biopsies were evaluated for presence of any glomerular disease, the type of glomerular disease, and the class of disease. The renal biopsies were evaluated by either of the two nephropathologists. Percentage of glomeruli with crescent formation was reported as none, 1%–50%, and >50%. Those glomeruli having >50% crescents were defined as crescentic glomerulonephritis (CrGN). The percentage of globally sclerosed glomeruli was noted and classified as none, 1%–50%, and >50%. Interstitial fibrosis and tubular atrophy (IFTA) was classified as none- 0%, mild- <25%, moderate- 25%–50%, and severe- >50%.
IgA nephropathy (IgAN) was defined as glomerular disease with IgA-dominant or co-dominant mesangial immunoglobulin deposits, excluding lupus nephritis (LN).[4] The cases were scored according to MEST scoring system. Proliferative group included those cases which had mesangial hypercellularity (M1), endocapillary proliferation (E1), and/or crescent formation (C1 and C2). C3 glomerulopathy (C3G) was defined as C3c intensity two orders of magnitude more than any other immunereactant on a scale of 1–4 (including 0, 1, 2, 3, 4).[5] Patients who presented with clinical features consistent with anti-Glomerular Basement Membrane (anti-GBM) disease in combination with positive glomerular linear IgG staining on immunofluorescence and/or positive serum anti-GBM antibodies were included in the study.[6] Histologic diagnoses were classified into one of three major categories: (1) primary glomerulopathy, (2) secondary glomerulopathy, and (3) hereditary glomerulopathy.
Data source and variables
Clinical data, laboratory data, and follow-up records of the patients were retrieved from the hospital information system. The treatment received by the patients was noted from the treatment charts. Patients ≤18 years of age were regarded as children.
Microscopic hematuria was defined as at least five red cells per high-power field on microscopic examination or positive blood by urine dipstick. Nephrotic syndrome was defined as nephrotic range proteinuria >3.5 g per 24 h per 1.73 m2 (in children, >40 mg/m2/h or protein creatinine ratio >2000 mg/g [>200 mg/mmol]) along with hypoalbuminemia and edema.[7] Acute nephritic syndrome was defined as the presence of hematuria (macroscopic or microscopic), proteinuria, edema, arterial hypertension, and acute kidney injury of variable degree.[8] Rapidly progressive glomerulonephritis (RPGN) is a clinical syndrome defined by the rapid loss (within days to weeks) of kidney function, accompanied by features of a nephritic syndrome along with proteinuria, glomerular hematuria, and often oliguria.[9] Advanced renal failure was used for patients fulfilling any of the following Kidney Disease Improving Outcomes Acute Kidney Injury (KDIGO-AKI) stage 3 criteria: 3.0 times baseline or initiation of renal replacement therapy or decrease in estimated glomerular filtration rate (eGFR) to <35 ml/min/1.73 m2.[10] End-stage renal disease (ESRD) was defined as a decrement in the patient’s kidney function to a level at which either long-term dialysis or kidney transplantation is required to sustain life.[11] CKD was defined as kidney damage or glomerular filtration rate (GFR) <60 ml/min/1.73 m2 for 3 months or more. eGFR was calculated using the updated Schwartz equation.[12]
The follow-up period was considered to be the time interval between renal biopsy and the last outpatient visit, death, or kidney failure, whichever happened earlier.
Statistical analysis
Statistical analysis was done using Statistical Package for the Social Sciences (SPSS) Version 23.0 (IBM Corp., Armonk, NY, USA). Categorical variables expressed as numbers or percentages were compared using Chi-square and Fisher’s exact test (as applicable). Continuous variables reported as mean or median (depending on the normality of data) were compared using Wilcoxon rank-sum methods.
Ethics approval and consent: This was not sought as it was a retrospective study. A local ethics committee ruled that no formal ethics approval was required in this particular case.
Results
A total of 2890 native renal biopsies were studied in 5 years, of which 422 were pediatric patients. Also, 2466 (85.3%) were diagnosed with glomerular diseases, of which 409 were children. The median age was 15 years (Interquartile Range 5), with the youngest patient being 4 years of age. Males outnumbered females, with an overall male: female ratio of 1.6:1. There was a significant male preponderance in minimal change disease (MCD) and membranous nephropathy (MN). Females outnumbered males in only LN. One hundred fifteen patients were young children and 294 were adolescents.
Clinical presentation
Pure nephrotic syndrome (NS) was the most common presentation (60.8%). Non-nephrotic proteinuria with hematuria was seen in 18.5% of biopsied cases. Nephritic syndrome with nephrotic range proteinuria was found in 1.2%, isolated hematuria in 5.3%, acute nephritic syndrome in 3.4%, non-nephrotic proteinuria in 1.9%, and rapidly proliferative glomerulonephritis (RPGN) in 7% of the cases. Advanced renal failure was observed in only 0.7% of biopsied cases.
Histopathologic diagnosis
There were 353 (86.3%) cases of primary glomerulonephritis, 51 (12.5%) cases of secondary glomerulonephritis, and five cases (1.2%) of hereditary glomerulonephritis [Figure 1]. The most common histologic diagnosis was MCD seen in 145 patients (35.5%), followed by focal segmental glomerulosclerosis (FSGS; 71, 17.4%), IgAN (41, 10%), MN (27, 6.6%) CrGN (12, 2.9%), and C3G (12, 2.9%). Among patients with CrGN, six (50%) were immune complex–mediated, five (41.6%) were anti-neutrophilic cytoplasm antibody–associated, and one (8.3%) was anti-glomerular basement membrane antibody–associated glomerulonephritis. Proliferative glomerulonephritis (immune complex–mediated membranoproliferative glomerulonephritis [MPGN], immune complex–mediated diffuse proliferative glomerulonephritis [DPGN], and mesangioproliferative glomerulonephritis (MesPGN) with no specific etiologies) was found in 50 patients (12.2%). LN was seen in 24 (5.9%) patients, post-infective glomerulonephritis in 11 (2.7%), and Henon–Schonlein Purpura (HSP) in two (0.5%) patients. Thrombotic microangiopathy was seen in nine (2.2%) patients.
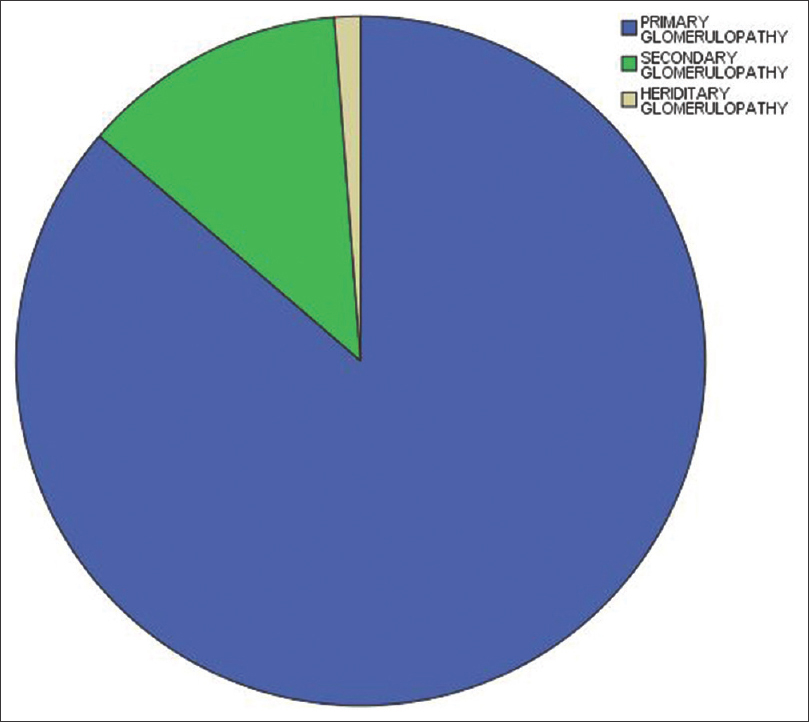
- Pi chart showing distribution of primary, secondary, and tertiary glomerulonephritis in our pediatric population
Hereditary nephritis included Alport’s disease (2, 0.5%), thin basement membrane disease (TBMD; 2, 0.5%), and Fabry’s disease (1, 0.2%). There were two patients of Alport’s syndrome, both being males. Only one had a positive family history along with abnormal vision and hearing. EM was available for 72 cases. EM was essential in the definitive diagnosis of Alport’s syndrome, TBMD, Fabry’s disease, and dense deposit disease.
Clinicopathologic correlation
Among the 252 patients with nephrotic syndrome, 143 had MCD, 66 patients had FSGS, and 23 had MN. Out of 10 patients with nephritic syndrome and nephrotic range proteinuria, three had DPGN, two had C3G, and two patients had IgAN. Most common diagnoses in the patients presenting with non-nephrotic proteinuria and hematuria included DPGN (18), IgAN (16), and LN (16). Out of 22 children with persistent isolated hematuria, IgAN was observed in 10 patients, non-IgA mesangioproliferative glomerulonephritis (MesPGN) in eight, and Alport’s syndrome in one patient. There were two patients of TBMD, both of which had isolated hematuria. Acute nephritic syndrome most commonly occurred in postinfectious glomerulonephritis (PIGN), and all patients of CrGN presented as RPGN. Three patients with advanced renal failure had IgAN (in two patients) and FSGS (in one patient). The clinical and histological correlation of pediatric patients in our study is shown in Table 1.
| Histological diagnosis | Clinical presentation | Total number | |||||||
|---|---|---|---|---|---|---|---|---|---|
| H | H+P | Ni/NS | NiS | NS | P | AdRF | RPGN | ||
| Alport’s disease | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| C3G | 0 | 3 | 2 | 0 | 4 | 3 | 0 | 0 | 12 |
| CrGN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 12 |
| DPGN | 0 | 18 | 3 | 1 | 0 | 0 | 0 | 4 | 26 |
| Fabry’sdisease | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| FSGS | 0 | 4 | 0 | 0 | 66 | 0 | 1 | 0 | 71 |
| HSP | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| IgAN | 10 | 16 | 2 | 4 | 4 | 1 | 2 | 2 | 41 |
| LN2 | 1 | 16 | 1 | 0 | 2 | 2 | 0 | 2 | 24 |
| MCD1 | 0 | 2 | 0 | 0 | 143 | 0 | 0 | 0 | 145 |
| MesPGN | 8 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 13 |
| MN | 0 | 4 | 0 | 0 | 23 | 0 | 0 | 0 | 27 |
| MPGN | 0 | 4 | 0 | 0 | 6 | 1 | 0 | 0 | 11 |
| PIGN | 0 | 1 | 0 | 8 | 0 | 0 | 0 | 2 | 11 |
| TBM | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| TMA | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 6 | 9 |
| TOTAL | 22 | 76 | 8 | 14 | 249 | 8 | 3 | 29 | 409 |
AdRF=advanced renal failure, C3G=C3 glomerulopathy, CrGN=crescentic glomerulonephritis, DPGN=diffuse proliferative glomerulonephritis, FSGS=focal segmental glomerulosclerosis, H=hematuria, H+P = non-nephrotic proteinuria with hematuria, HSP=Henon-Schonlein purpura, IgAN=IgA nephropathy, LN=lupus nephritis, MCD=minimal change disease, MesPGN=mesangioproliferative glomerulonephritis, MN=membranous nephropathy, MPGN=membranoproliferative glomerulonephritis, Ni/NS=nephritic syndrome with nephrotic range proteinuria, NiS=nephritic syndrome, NS=nephrotic syndrome, P=isolated proteinuria, PIGN=postinfectious glomerulonephritis, RPGN=rapidly proliferative glomerulonephritis, TBM=thin basement membrane disease, TMA=thrombotic microangiopathy. 1The bold font marks the most common primary glomerular disease. 2The bold font marks the most common secondary glomerular disease
There were no major differences in the clinicopathologic correlations between males and females.
Adolescents versus young children
Nephrotic syndrome was the most common presentation in both adolescents (168, 57.1%) and young children (81, 70.4%). Non-nephrotic proteinuria with hematuria was more commonly seen in adolescents (63, 21.4%) compared to younger children (13, 11.3%). The same was true for isolated hematuria. All three children with advanced renal failure belonged to the adolescent category. Table 2 shows the clinical presentation of patients according to their age group. Although MCD was the most common glomerular disease in both the age groups, the incidence was significantly higher in young children (47.8% vs. 30.6%). On the other hand, the incidence of IgAN (4.3% vs. 12.2%), MN (4.3 vs. 7.4), and DPGN (2.6 vs. 7.4%) was significantly higher in adolescents than in younger population.
| Age group | Clinical presentation | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| H | H+P | Ni/NS | NiS | NS | P | AdRF | RPGN | ||
| Young children | 3 (2.6%) | 13 (11.3%) | 1 (0.8%) | 4 (3.4%) | 81 (70.4%) | 4 (3.4%) | 0 (0) | 9 (7.8%) | 115 |
| Adolescents | 19 (6.4%) | 63 (21.4%) | 7 (2.3%) | 10 (3.4%) | 168 (57.1%) | 4 (1.3%) | 3 (1.0%) | 20 (6.8%) | 294 |
AdRF=advanced renal failure, H=hematuria, H+P = non-nephrotic proteinuria with hematuria, Ni/NS=nephritic syndrome with nephrotic range proteinuria, NiS=nephritic syndrome, NS=nephrotic syndrome, P=isolated proteinuria, RPGN=rapidly proliferative glomerulonephritis
Follow-up
Most children were on regular follow-up. A total of 218 (53.3%) improved and were discharged from follow-up, 135 (33%) remained on follow-up, 35 (8.5%) were lost to follow-up, 12 (2.9%) progressed to CKD, and nine (2.2%) died. All patients who were lost to follow-up were children from remote areas or who had financial constraints.
Discussion
Children are not mini adults when it comes to glomerular diseases. In fact, in addition to age, the pattern varies according to the gender, race, geographic area, and environmental factors.[13] Our study provides detailed information about the biopsy-proven glomerular diseases in children in a homogenous Asian population in North India. Also, adolescents form a subgroup different from younger children and their differences from the younger subgroup have not been studied in detail. This study highlights the major differences between the two subgroups.
The glomerular diseases in children comprise 16.5% of the total patients who underwent renal biopsy, predominantly affecting males with a median age of 15 years (ranging from 4 to 18 years). The mean age widely varies in literature and ranges from 9[14] to 15[15] years. In one of the largest studies from China, the average age was 13.5 ± 4.1 years.[2] A major percentage of our patients were adolescents (72.3%), which is consistent with other studies. This is mostly attributed to better acceptability and lesser threshold of performing renal biopsy in older children.[2]
Almost all other studies have shown a male predominance in pediatric glomerular diseases, with females outnumbering males only rarely, as in LN. The overall male predominance has been attributed to higher susceptibility of boys to the most frequent glomerular diseases like MCD, FSGS, and IgAN.[2]
The most common clinical presentation was nephrotic syndrome seen in 60.8% of the patients, followed by non-nephrotic proteinuria and hematuria. Although nephrotic syndrome has been the predominant symptom in many studies from Iran[16] and China,[2,17] there are reports from UK where non-nephrotic proteinuria predominated the picture.[18] In our study, isolated hematuria was the fourth common indication for renal biopsy, whereas Coppo et al.[19] and Rychlik et al.[20] found that isolated hematuria was the commonest key indication for renal biopsy in children.[21] The second common symptom was also not consistent. Non-nephrotic proteinuria with hematuria was present in 18.5% of our patients. A study from southern Croatia also showed non-nephrotic proteinuria with hematuria as the second most common indication. While studies from China,[2] Iran,[16] Sudan,[15] and many Arab countries have shown acute nephritic syndrome as the second most common symptom, asymptomatic hematuria was much more common in Serbia[22] and abnormal urinary sediment in Morocco.[14]
Primary glomerular diseases predominated the picture, accounting for 86.3% of the total cases. The most predominant symptom in our study correlated with the most predominant histology, as MCD was the first and FSGS was the second most common glomerular disease. The spectrum of glomerular diseases in children shows variation between different countries. Many studies from Iran,[23] China,[2] some North African countries like Sudan[13] and Morocco,[14] and few Arab countries[15] have shown MCD as the commonest pathological diagnosis in renal biopsies. However, other studies from European countries like Croatia[21] and from some regions of China,[24] Korea,[25] and Italy[19] have shown IgAN as the most common histopathologic diagnosis. HSP was the most common histopathologic diagnosis in London,[18] whereas FSGS was most common in Serbia,[22] Europe,[14] and USA.[26-28] In a previous study from northwest India, MesPGN was the commonest histopathologic diagnosis.[29]
Nephrotic syndrome was the most common presentation among children with MCD (97.3%) and FSGS (94.3%). There were no statistically significant clinical differences between our patients of FSGS and MCD. One of the reasons for this may be that this study included only the biopsied cases. Typical cases of steroid-sensitive MCD were not biopsied. MCD was the most common histological diagnosis for children presenting with nephrotic syndrome in many other studies as well. There have been reports from USA, Brazil, and India that show an increasing trend for idiopathic FSGS in the 1980s, 1990s, as well as 2000s.[24] The increase in incidence of FSGS from USA and Europe has been speculated to be related to a recent worldwide epidemic of obesity by some authors.[22] However, on the contrary, studies from China[2] and Arab countries[15] observed significant decreasing rates over time. Some authors think that this decline in incidence of FSGS may not be a true decrease, but may be due to a relative increase in biopsies for other indications. However, the exact reason for the same is not known.
LN, DPGN, and MesPGN were the common histological diagnoses in patients of non-nephrotic proteinuria with hematuria. In Croatia and Japan,[21] IgAN was the most usual diagnosis in patients with non-nephrotic proteinuria and hematuria, whereas in a Chinese study, HSP nephritis (25%) was the most common etiology of proteinuria without nephrotic syndrome, followed by MesPGN (23%) and LN (16%).[2] Isolated hematuria was most commonly seen in IgAN. Though MesPGN was most commonly seen in pediatric patients presenting as isolated hematuria in studies from China,[2] IgAN was the most frequent histopathologic diagnosis in Croatia and Italy[21] and systemic lupus erythematosus (SLE) nephritis in Iran.[23] Thus, children with non-nephrotic proteinuria with hematuria as well as isolated hematuria were more likely to have glomerulonephritis as their histopathologicdiagnosis.
Acute nephritic syndrome was most commonly seen in PIGN in our study. Among patients with clinical presentation of acute glomerulonephritis, HSP nephritis was the most frequent cause, followed by SLE nephritis, MPGN, and endocapillary glomerulonephritis (ECGN) in Iran. The low frequency of PIGN in other studies may be because patients with “typical” PIGN were not biopsied at all.[23]
Among our three patients with advanced renal failure, two had IgAN and one had FSGS. These two conditions were the lead causes in patients with CKD in other studies as well.[2,21] The lack of data in the chronic renal failure group could be the reason for its small numbers in children and also the fact that many patients with advanced stage of CKD are not biopsied.[21] LN was the most common secondary glomerular disease in our study. HSP nephritis and LN (9%) were the two most common secondary glomerular diseases[2,21,23,29,30] in other studies as well.
These differences in the pattern of glomerular diseases in children may be multifold. The most obvious reason is that there are no standard guidelines for renal biopsy in pediatric patients. Also, patient referral protocols may vary, accounting for the variations among different institutes in a single region. There is variation among set protocols not only indifferent countries, but also in different states of a single country like India. Low frequency of IgAN and HSP nephritis in our study may be due to lack of routine urinary dipstick screening programs in our country compared to the West. The other reasons include racial and environmental factors.
On comparison of data between adolescents and young children, DPGN, IgAN, and MN were more commonly seen in adolescents. In our study, though the percentage of MCD was greater in younger children than in adolescents, it was the most common diagnosis in adolescents also. This was in contrast to studies from other parts of India where FSGS was the most common histopathologicdiagnosis in adolescents,[1] but in line with a nation-wide study from China where MCD (33%) was the most common glomerulopathy in adolescents (13–18 years old).[2] IgAN and MN were significantly higher in adolescents than in younger children in other studies as well.[2] The histological diagnosis pattern correlates with the clinical presentation where isolated hematuria and proteinuria with hematuria were more commonly seen in adolescents than in younger children. Similar to other studies, though nephrotic syndrome predominated in all ages, it gradually deceased with age.[31]
EM was not done in all the cases. It was needed for the diagnosis of hereditary nephritis like Alport’s disease, TBMD, and Fabry’s disease. If only viewed through light microscopy and immunofluorescence, these cases would have been classified into MCD or FSGS. EM was also essential in our cases of C3G, which were further subdivided into C3 glomerulonephritis and dense deposit disease. Subepithelial humps, seen in cases of immune complex/complement-dominant proliferative nephritis, helped in classifying these disorders into PIGN, along with the clinical history and follow-up. In the remaining cases, EM only had a supportive role. EM, although not essential in all cases of nephrotic syndrome, should be done in cases where presentation is not typical. Patients with syndromic presentation, family history of hereditary nephritis, benign hematuria, and suspected PIGN should be evaluated with it. EM is also useful in cases where there is a disparity between histopathology report and clinical history. The role of EM in the final diagnosis of pediatric renal biopsies was studied in only a few studies which have shown a wide range in which EM was essential (ranging from 31% to 63%). However, we could not come to any definite numbers due to the fact that EM was not performed in all our cases. Further extensive studies are needed to quantify the exact role of EM in pediatric renal cases.[32–34]
Strengths
The main strengths of this study were the large sample size and the use of EM for diagnosis of hereditary nephritis cases.
Limitations
The selection bias of the study population, which is inevitable in all retrospective biopsy-based studies, occurred in this study also. Also, this is not a nation-wide study and institutional protocols affected the outcome. This was a retrospective study, so changes in treatment received and their impact on prognosis could not be tracked properly.
Conclusions
In younger patients, even in children who are biopsied selectively, MCD remains the most common histopathologic diagnosis. LN is the most common secondary glomerulopathy in our pediatric patients. The adolescent-onset glomerular diseases have a higher frequency of histopathology other than MCDs like IgAN, MN, and DPGN, as well as a higher percentage of microscopic hematuria, compared to the younger children. Also, despite decreasing trends of postinfectious glomerular diseases in other countries, it is still an important differential in India. A national kidney biopsy registry along with clinical information and long-term follow-up is required for accurate estimation of pediatric diseases and their natural history in India.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Clinicopathological spectrum of glomerular diseases in adolescents: A-single-center experience over-4-years. Indian J Nephrol. 2018;28:15-20.
- [Google Scholar]
- The spectrum of biopsy-proven glomerular diseases among children in China: A-national, cross-sectional survey. Clin J Am Soc Nephrol. 2018;13:1047-54.
- [Google Scholar]
- Chronic kidney disease in children: An Indian perspective. Med J Armed Forces India. 2009;65:45-9.
- [Google Scholar]
- IgA nephropathy in a tertiary care center from south India. Indian J Nephrol. 2011;21:230-4.
- [Google Scholar]
- C3 glomerulonephritis and dense deposit disease share a similar disease course in a large United States cohort of patients with C3 glomerulopathy. Kidney Int. 2018;93:977-85.
- [Google Scholar]
- Clinicopathological characteristics of typical and atypical anti-glomerular basement membrane nephritis. J-Nephrol. 2017;30:503-9.
- [Google Scholar]
- Kidney disease. Improving global outcomes-(KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. 2012;2:139-274.
- [Google Scholar]
- New trends of an old disease: The acute post infectious glomerulonephritis at the beginning of the new millenium. J Nephrol. . 2014;27:229-39.
- [Google Scholar]
- What is new in the management of rapidly progressive glomerulonephritis? Clin Kidney J. 2015;8:143-50.
- [Google Scholar]
- Kidney Int Suppl. 2011;2:19-36.
- Defining end-stage renal disease in clinical trials:Aframework for adjudication. Nephrol Dial Transplant. 2015;31:864-7.
- [Google Scholar]
- Measurement and estimation of glomerular filtration rate in children. Adv Chronic Kidney Dis. 2017;24:348-56.
- [Google Scholar]
- Pattern of glomerular diseases in Sudanese children:-A clinico-pathological study. Saudi J Kidney Dis Transpl. 2010;21:778-83.
- [Google Scholar]
- Indications and results of renal biopsy in children: A-single-center experience from Morocco. Saudi J Kidney Dis Transpl. 2015;26:810-5.
- [Google Scholar]
- Incidence of pediatric glomerular diseases in Arab world: A-systematic review. Saudi J Kidney Dis Transpl. 2019;30:15-23.
- [Google Scholar]
- Clinicopathologic characteristics of 1,316 children with renal disease. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9:117-21.
- [Google Scholar]
- Renal biopsies in children:Current practice and audit of outcomes. Nephrol Dial Transplant. 2010;25:485-9.
- [Google Scholar]
- Frequency of renal diseases and clinical indications for renal biopsy in children-(report of the Italian National Registry of renal biopsies in children). Group of RenalImmunopathology of the Italian Society of Pediatric Nephrology and Group of renal immunopathology of the Italian Society of Nephrology. Nephrol Dial Transplant. 1998;13:293-7.
- [Google Scholar]
- The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994-2000. Nephrol Dial Transplant. 2004;19:3040-9.
- [Google Scholar]
- Epidemiology of 10-year paediatric renal biopsies in the region of southern Croatia. BMC Nephrology. 2020;21:65.
- [Google Scholar]
- Indications and results of renal biopsy in children:A10-year review from a single center in Serbia. J-Nephrol. 2012;25:1054-9.
- [Google Scholar]
- Glomerular diseases in Iranian children:Clinico-pathological correlations. Pediatr Nephrol. 2003;18:925-8.
- [Google Scholar]
- The changing spectrum of primary glomerular diseases within 15-years:A-survey of 3331-patients in a single Chinese centre. Nephrol Dial Transplant. 2009;24:870-6.
- [Google Scholar]
- A-nationwide study of mass urine screening tests on Korean school children and implications for chronic kidney disease management. Clin Exp Nephrol. 2013;17:205-10.
- [Google Scholar]
- Outcomes of kidney transplantation in children with nephronophthisis:An analysis of the North American Pediatric Renal Trials and Collaborative Studies-(NAPRTCS) Registry. Pediatr Transplant. 2008;12:878-82.
- [Google Scholar]
- Increasing incidence of focal segmental glomerulosclerosis and an examination of demographic patterns. Clin Nephrol. 2005;63:1-7.
- [Google Scholar]
- Distribution of biopsy-proven presumed primary glomerulonephropathies in 2000-2011 among a racially and ethnically diverse US population. Am J Kidney Dis. 2016;68:533-44.
- [Google Scholar]
- Pediatric renal biopsies in India:A-single-centre experience of six years. Nephrourol Mon. 2015;7:e25473.
- [Google Scholar]
- Changing trends in pediatric renal biopsies:Analysis of pediatric renal biopsies in national nephrology registry data. Ren Fail. 2016;38:1228-33.
- [Google Scholar]
- Twenty-three-year review of disease patterns from renal biopsies:An experience from a pediatric renal center. J Nephrol. 2013;26:699-707.
- [Google Scholar]
- Value of electron microscopy in the pathological diagnosis of native kidney biopsies in children. Pediatr Nephrol. 2020;35:2285-95.
- [Google Scholar]
- Role of electron microscopy in the diagnosis of nonneoplastic renal disease in children. UltrastructPathol. 2011;35:240-4.
- [Google Scholar]
- Study of nephrotic syndrome in children:Importance of light, immunoflourescence and electron microscopic observations to a correct classification of glomerulopathies. Nefrologia. 2013;33:237-42.
- [Google Scholar]







