Translate this page into:
Plasmablastic myeloma presenting as rapidly progressive renal failure in a young adult
Address for correspondence:Dr. V. N. Unni, Department of Nephrology, Amrita School of Medicine, Amrita Institute of Medical Sciences and Research Centre, Kochi - 682 041, Kerala, India. E-mail: unnivn@aims.amrita.edu
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Multiple myeloma (MM) is a condition where there is malignant proliferation of plasma cells. There is a strong correlation with age, peaking at 60-70 years. The clinical course in adolescents and young individuals is generally indolent and the survival is longer. We report a case of a 28-year-old male, who was diagnosed to have plasmablastic myeloma, an atypical variant of MM with a poor prognosis, presenting as rapidly progressive renal failure. He was given induction chemotherapy and then underwent autologous peripheral blood stem cell transplantation.
Keywords
Multiple myeloma in young
plasmablastic myeloma
rapidly progressive renal failure
Introduction
Multiple myeloma (MM) is a clonal plasma cell proliferative disorder, that accounts for 1% of all malignancies and 10% of malignant hematologic neoplasms.[1] It is a disease with a higher incidence in the elderly, with a male predominance and is extremely rare in young adults below 30 years of age.[23] Even though, it is considered as a single disease, there are many clinical entities (monoclonal gammopathy of undetermined significance, smoldering MM, Waldenstrom's macrog lobulinemia, solitary plasmacytoma, systemic amyloidosis, POEMS syndrome) and cytogenetically distinct entities (translocation of genes (11;14), t (4;14), t (14;16), t (6;14), t (14;20), hyperdiploidy and deletion of 17p), that are currently lumped together; hence, there is an element of subjectivity involved in interpreting the clinical criteria for diagnosis and hence, deciding on therapeutic requirements.
The cytomorphologic features of plasma cell tumors ranges from mature, atypical to plasmablastic or anaplastic plasma cells. Plasmablastic MM is a morphologic subset of myeloma, in which the bone marrow (BM) aspirate slide stained with Wright stain shows ≥2% of plasmablasts. Plasmablasts are characterized by fine reticular nuclear chromatin pattern, large nucleus (greater than 10 μm), large nucleolus (greater than 2 μm), cytoplasm with no or very little hof region (area containing Golgi apparatus) and less abundant cytoplasm (less than one half of the nuclear area).[4] The prevalence of plasmablastic morphology in patients with newly diagnosed myeloma is approximately 10%. Many of the previous western studies have shown that plasmablastic cytomorphologic features of the neoplastic plasma cells in marrow aspirate strongly correlate with poor survival. Eastern cooperative oncology group has recently confirmed that plasmablastic morphology is a powerful independent adverse prognostic factor for survival.[5]
We report a case of a 28-year-old male patient who was diagnosed to have plasmablastic myeloma, considered to be an atypical variant of MM with poor prognosis, presenting as rapidly progressive renal failure; the outcome in this patient following treatment was extremely gratifying.
Case Report
A 28-year-old male patient presented with the complaints of generalized weakness and exertional dyspnea of 1 month duration. He did not give a history of frothing of urine, pedal edema, hematuria or breathlessness in the past. Clinically he appeared pale and dehydrated, blood pressure was 120/80 mmHg and systemic examination was unremarkable. Investigations revealed that he had normocytic normochromic anemia (hemoglobin: 7.5 g/dl), thrombocytopenia (80,000/cumm) with normal leukocyte count (7400/cumm), moderate renal failure (serum creatinine: 3 mg/dl), normal liver function tests (serum albumin: 4.92 g/dl and serum globulin: 2.4 g/dl), and normal serum electrolytes, serum calcium: 10 mg/dl, serum phosphorous: 4.7 mg/dl and elevated serum uric acid (9.5 mg/dl). Urine examination showed proteinuria 1+ on dipstick, 24 h urine protein was 8504 mg. With a high index of suspicion for a tubular proteinuria, along with a hypo proliferative marrow (reticulocyte productive index: 0.7%), the possibility of plasma cell dyscrasia was considered. Serum protein electrophoresis and urine Bence Jones protein were negative. Serum immunofixation using capillary method showed immunoglobulin G, kappa monoclonal immunoglobulin. Urine electrophoresis showed M band and serum free light chain (SFLC) assay showed a significantly elevated kappa level (4383 mg/l; normal range: 3.3-19.4) with a suppressed lambda level (4.5 mg/l; normal range: 5.7-26.3), kappa/lamba ratio was 974 (normal range in renal failure: 0.37-3.1). Skeletal survey did not show any evidence of lytic lesions. BM biopsy done showed 79% of plasma cells with plasmablastic morphology [Figure 1], which were CD138 positive with kappa restriction. These atypical cells showed Ki 67% of 20-25% with very few cells positive for terminal deoxynucleotidyl transferase and CD79a was negative, which suggested plasmablastic myeloma phenotype. Fluorescence in-situ hybridization on BM was negative for del13q14.3, t (11;14), t (4;14), t (14;16). Renal function deteriorated rapidly; he underwent a renal biopsy, which showed pale eosinophilic, Schiff poor fractured casts in the renal tubules [Figure 2], which were kappa restricted.
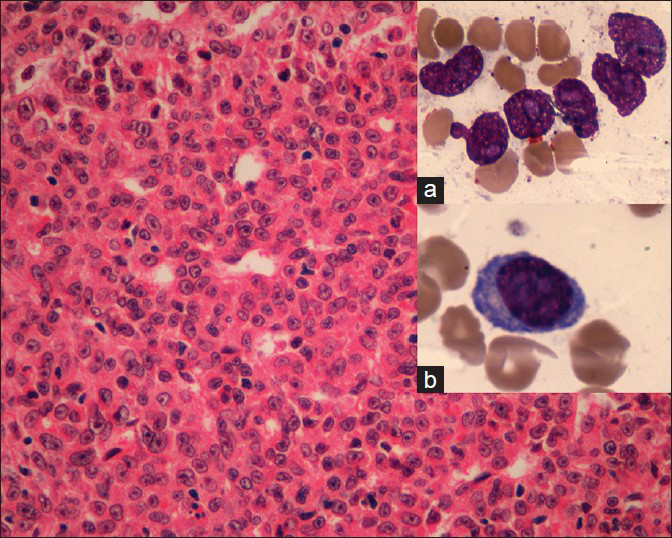
- Bone marrow biopsy (H and E, ×100) showing plenty of atypical plasma cells; with bone marrow aspirate (H and E, ×400) showing plasmablasts (insets a and b)
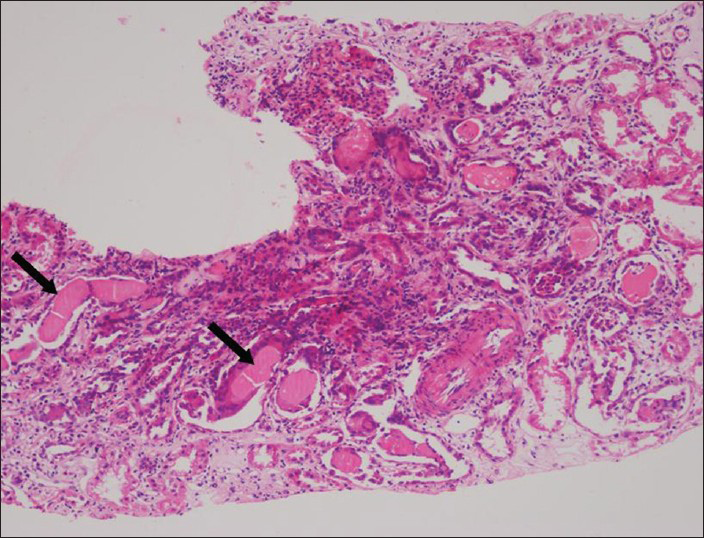
- Renal biopsy (H and E, ×100) showing interstitial infiltrates with mononuclear cells and extensive tubular pale eosinophilic fractured casts (arrows), which were kappa restricted on immunoflurosence
He was started on chemotherapy, with each 21 days cycle consisting of parenteral bortezomib (1.3 mg/m2/dose on days 1, 4, 8 and 11) and oral dexamethasone (40 mg/day on days 1-4, 9-12). After the first cycle of bortezomib and dexamethasone, his renal functions started to improve. He was started on lenalidomide 25 mg/day at cycle 2 (when renal function started to improve) along with bortezomib and dexamethasone. With this intensive chemotherapy, his renal functions improved further (serum creatinine 1.77 mg/dl at 6 weeks).
Patient was reevaluated after four cycles of chemotherapy with BM examination and free light chain assay. BM showed less than 1% of plasma cells and serum free kappa light chain was 67.69 mg/l. Serum creatinine improved to 1.4 mg/dl at 6 months and he was planned for autologous peripheral blood stem cell transplantation (APBSCT). However, due to technical delay, he was given additional two cycles of CyBorD chemotherapy (weekly cyclophosphamide, bortezomib and dexamethasone). Patient then underwent APBSCT with Melphalan conditioning 140 mg/m2. Post-transplant patient achieved neutrophil and platelet engraftment on day +9 and did not encounter any infections. Day +100 evaluation showed serum creatinine to be 1.3 mg/dl and he was in stringent complete remission (negative serum and urine immunofixation and normal SFLC ratio).
Discussion
Traditionally, the diagnosis of MM requires 10% or more clonal plasma cells in the BM or the presence of a biopsy proven plasmacytoma, plus evidence of end-organ damage (anemia, hypercalcemia, lytic bone lesions, or renal failure) attributable to the underlying plasma cell disorder.[6] Predominately a disease of the elderly, the peak age incidence is between 60 and 70 years; the occurrence of MM in patients younger than 30 years of age is rare and they may have an atypical clinical presentation and an indolent course with prolonged survival.[7] Five patients with MM of less than 30 years of age have been reported from India, during the last 10 years, which constituted 3.3% of all MM cases; and none of these young patients described above had renal failure.[7] Review of a series of case reports of myeloma in young patients (youngest being a 10-year-old girl), revealed that most of them presented with solitary plasmacytoma.[89] Our patient is a young male who presented with rapidly progressive renal failure.
The pathophysiology of acute kidney injury in MM is multi-factorial. Renal injury in the majority of cases is attributed to tubulointerstial damage, which results in myeloma kidney as a direct consequence of the high SFLC levels.[10] Other causes include dehydration, hypercalcemia, hyperuricemia, amyloid deposition, infections and exposure to nephrotoxic medications. SFLC are primarily metabolized in the proximal tubule by endocytosis and subsequent degradation within the lysosomes. When surplus amounts of free light chains are produced, as in the case of MM, the absorptive mechanisms are overwhelmed in the proximal tubules and the light chains enter the distal tubules and subsequently appear as Bence Jones proteins in urine. Bence Jones proteins interact with Tamm Horsfall protein in the distal tubule, resulting in tubular cast formation with giant cell reaction. This results in myeloma kidney or myeloma cast nephropathy. Dipstick methods for detecting proteinuria for recognizing free light chains are unreliable; and conventional tests used for detecting Bence Jones proteins are falsely negative in approximately one-half of patients with light chain MM.[11] Immunoelectrophoresis of concentrated urine is the methods of choice for detection of a monoclonal light chain in the urine.[12]
Although the median duration of survival of patients with MM ranges between 2 and 3 years, survival of the younger patients is considerably longer.[13] General measures advised include avoidance of nephrotoxic drugs, adequate hydration, correcting hypercalcemia, hyperuricemia and avoidance of radio contrast agents. These standard management principles are often successful in correcting moderate degrees of renal injury. The specific regimen chosen for initial therapy varies widely, but in general high dose chemotherapy with autologous stem cell transplantation (ASCT) may be an effective option in younger patients because of better tolerance to conditioning regimes with good long lasting responses. High-dose chemotherapy before ASCT improves survival in selected patients.[1415] Recent evidence suggests that therapies with novel agents (lenalidomide, bortezomib) have increased the survival of patients.[1316] Initial therapy is decided based on eligibility for ASCT. Patients eligible for ASCT are treated with a non-Melphalan containing induction regimen followed by transplantation and patients who are not eligible for ASCT have traditionally been treated with a Melphalan containing regimen for 12-18 months.[17] The options for the treatment of relapsing disease have increased, with ASCT remaining the cornerstone of therapy for appropriately selected patients; the duration of remission decreases with each salvage regimen.[18]
To the best of our knowledge, a case of plasmablastic myeloma with cast nephropathy presenting as rapidly progressive renal failure in a young male, has not been reported before. Occurrence of atypical myeloma with an atypical presentation in a young person is rare and has not been yet reported in the literature. The severe renal failure at presentation could have been presumed to be a primary glomerular disease, but for the high index of suspicion of tubular proteinuria, which paved the way for diagnosis and was subsequently confirmed with renal histology.
Conclusion
A possibility of MM should be considered in patients with unexplained renal failure, even if they are young. Early recognition and timely initiation of appropriate management is likely to result in a good response; recovery of renal functions may occur, if a significant reduction in the level of light chain load is achieved with chemotherapy.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Multiple myeloma in patients younger than 30 years. Report of 10 cases and review of the literature. Arch Intern Med. 1996;156:1463-8.
- [Google Scholar]
- Mature plasma cells as indicator of better prognosis in multiple myeloma. New methodology for the assessment of plasma cell morphology. Leuk Res. 1999;23:1133-40.
- [Google Scholar]
- Plasmablastic morphology – An independent prognostic factor with clinical and laboratory correlates: Eastern Cooperative Oncology Group (ECOG) myeloma trial E9486 report by the ECOG Myeloma Laboratory Group. Blood. 1998;91:2501-7.
- [Google Scholar]
- Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3-9.
- [Google Scholar]
- Clinical spectrum and prognosis of multiple myeloma in patients younger than 30 years: Is it different from the elderly? JK Sci. 2006;8:225-8.
- [Google Scholar]
- Multiple myeloma in young men. Clinical course and electron microscopic studies of bone marrow plasma cells. Cancer. 1980;46:1397-400.
- [Google Scholar]
- Management of myeloma-associated renal dysfunction in the era of novel therapies. Expert Rev Hematol. 2012;5:51-66.
- [Google Scholar]
- Urine protein electrophoresis and immunoelectrophoresis using unconcentrated or minimally concentrated urine samples. Am J Clin Pathol. 2008;130:141-5.
- [Google Scholar]
- Correlation of serum immunoglobulin free light chain quantification with urinary Bence Jones protein in light chain myeloma. Clin Chem. 2002;48:655-7.
- [Google Scholar]
- The role of autologous hematopoietic stem cell transplantation in multiple myeloma. Semin Hematol. 1997;34:61-6.
- [Google Scholar]
- A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335:91-7.
- [Google Scholar]
- Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516-20.
- [Google Scholar]
- Approach to the treatment of multiple myeloma: A clash of philosophies. Blood. 2011;118:3205-11.
- [Google Scholar]
- Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79:867-74.
- [Google Scholar]







