Translate this page into:
Podocyte Infolding Glomerulopathy Masquerading as Membranous Nephropathy – A Shared Pathogenesis?
Corresponding author: Kishore Gopalakrishnan, Department of Cellular Pathology, University Hospital Coventry and Warwickshire, Coventry, UK. E-mail: matthai.kishoregk@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Matthai SM, Hems L, Tsang YW, Aggarwal Y, Mahmoud H, Gopalakrishnan K. Podocyte Infolding Glomerulopathy Masquerading as Membranous Nephropathy – A Shared Pathogenesis? Indian J Nephrol. 2024;34:397-400. doi: 10.25259/ijn_209_23
Abstract
Podocyte infolding glomerulopathy (PIG) is a rare pathological entity, diagnosed by electron microscopic demonstration of diffuse infolding of the podocytes into the glomerular basement membranes. We report the first case from United Kingdom exhibiting typical ultrastructural features of PIG in a male with Type II diabetes mellitus, hypertension and common variable immune deficiency. Renal biopsy revealed phospholipase A2 receptor (PLA2R) immunostain positive membranous nephropathy (MN) but no serum PLA2R antibodies. Diffuse infolding of the podocytes into the glomerular basement membranes along with pathognomonic microspherular and microtubular intra basement membrane clusters distributed diffusely and globally were noted on electron microscopy, diagnostic of PIG. We postulate a shared pathomechanistic link between PIG and MN, highlighting the overlapping features of both conditions.
Keywords
Podocyte infolding glomerulopathy
Membranous nephropathy
Podocytopathy
Phospholipase A2 receptor
Electron microscopy
Introduction
Podocyte infolding glomerulopathy (PIG) has recently gained recognition as a novel diagnostic entity, possibly reflecting podocyte injury. Descriptions of PIG are limited to a few case reports, initially from Japan,1 followed by India,2 and then from various parts of the world.3-6 PIG is characterized by ultrastructurally demonstrable infolding of the podocyte cytoplasmic processes into underlying glomerular basement membranes (GBMs), along with clusters of microspherular/microtubular structures.1 Initially suspected to be a variant of Membranous nephropathy (MN) due to their frequent co-occurrence, the unique electron microscopy (EM) findings of PIG have led to it being considered a distinct entity.1,2,7 Nevertheless, the association between PIG and MN remains a source of speculation.
We describe a case fulfilling the diagnostic criteria of PIG with immunostain positive but serologically negative phospholipase-A2 receptor antibody (PLA2R) MN and discuss possible links in the pathogenesis.
Case Report
A 58-year-old male, on oral medication for type-2 diabetes mellitus and hypertension of five years, was investigated for nephrotic-range proteinuria. His urine Albumin: Creatinine (uACR) was 334 mg/gm. The serum creatinine was 0.79 mg/dL and the estimated glomerular filtration rate was 105 mL/min/1.73 m2 (CKD-EPI). Lipid profiles were normal, and HbA1c was 4.9. Hepatitis B, C, HIV, and routine immunology were normal except for pan-hypogammaglobulinemia (IgG 2.1 g/L (5.3-16.5), IgA 0.61g/L (0.80-4.00), IgM 0.15 g/L (0.50-2)). Complement, serum-free light chains, and Kappa: Lambda were normal with no detectable paraprotein. Serum PLA2R autoantibodies were negative.
Renal biopsy revealed 24 viable glomeruli with diffuse capillary wall thickening and no significant mesangial expansion, ruling out a diabetic contribution to renal injury. Focal thickened capillary walls showed a ‘hole and spike’ appearance on the Jones Silver-Methenamine stain [Figure 1a]. Minimal interstitial fibrosis, hyaline arteriolosclerosis, and mild fibro-intimal thickening of arteries were noted.
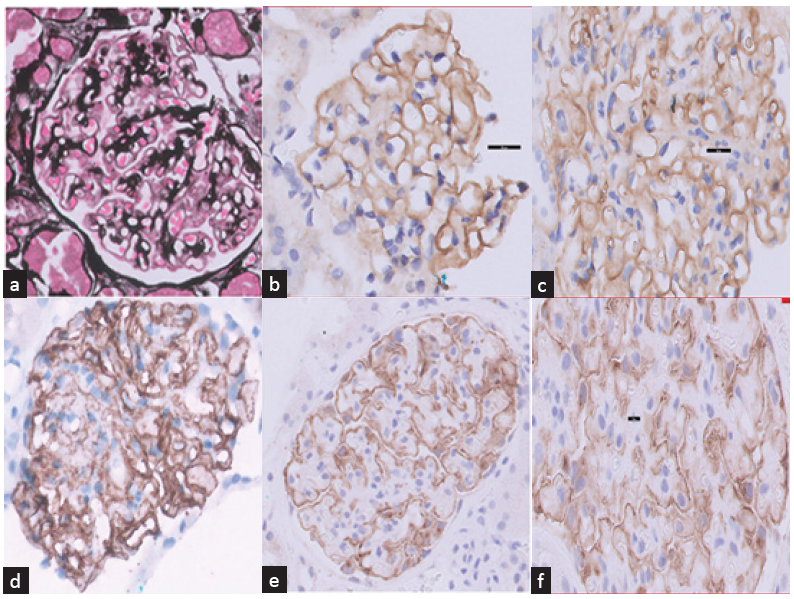
- (a) Glomerulus showing irregularly thickened capillary walls with bubbly appearance consistent with MN (Jones silver stain x400). (b) Weak positivity (1+) for IgG on glomerular capillary walls (IHC IgG x800). (c) Moderate positivity (2+) for C3 on glomerular capillary walls (IHC C3 x800). (d) Strong positivity (3+) for C4d on glomerular capillary walls and few podocytes (IHC C4d x800). (e) Diffuse strong and granular staining on capillary walls with PLA2R immunostain (IHC PLA2R x400). (f) Strong granular positivity (3+) for PLA2R on glomerular capillary walls (IHC PLA2R x1000). PLA2R: Phospholipase A2 Receptor Antibody, IHC: Immonohistochemistry.
Immunohistochemistry (IHC) showed weak granular deposition of IgG (1+) on capillary loops, moderate staining for C3 (2+), strong staining for C4d (3+) [Figure 1b-d], and negative staining for IgA. Strong diffuse reactivity was observed on capillary walls for PLA2R immunostaining [Figure 1e-f].
EM revealed diffuse thickening and lamellation of GBMs with numerous microspherical/microtubular particles within the GBM [Figure 2] with no demonstrable electron-dense deposits. Podocyte foot processes were diffusely effaced with focal invagination of podocyte cytoplasmic membranes into the distorted GBM, consistent with a diagnosis of PIG.
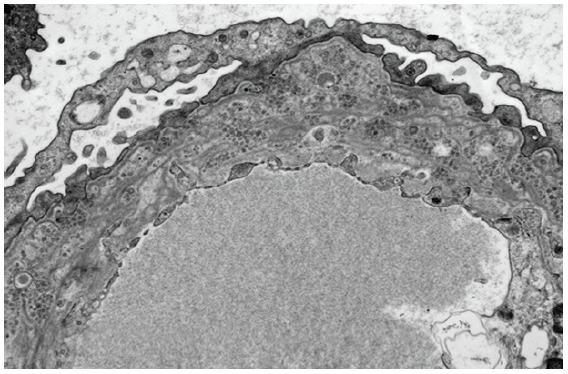
- Electron microscopic image showing characteristic microspherical particles entrapped within a lamellated glomerular basement membrane (TEM uranyl acetate stain x3000).
A multi-disciplinary decision was taken to treat MN in the context of idiopathic pan-hypogammaglobulinemia with four months of Prednisolone 0.75mg/kg monotherapy daily and prophylactic azithromycin. On completion of treatment, uACR dropped to 28.8mg/gm. However, maintenance immunosuppression was not advised as immunoglobulins continued to decline. He has remained under review for the last two years and was diagnosed with common variable immune deficiency after the first year, but his proteinuria has been in remission.
Discussion
We describe PIG occurring in association with PLA2R immunostain-positive MN without detectable serum PLA2R autoantibodies, throwing open the possibility of a shared pathogenesis pathway.
To date, 44 cases of PIG have been reported in the literature1-7 with a predominant association with autoimmune disorders. This suggests that the initiating step in the pathogenesis of PIG is likely to be podocyte injury due to autoantibodies targeting podocyte-expressed antigens, of which PLA2R is one, similar to the pathogenesis of MN. We propose that immune-mediated targeting of podocyte antigens such as PLA2R, leading to in situ glomerular immune-complex deposition, manifests as primary MN, while similar targeting resulting in podocyte injury and infolding manifests as PIG with or without detectable immune complexes [Figures 2 and 3]. It is also conceivable that there are independent podocyte injury pathways operating in conjunction, which may overlap in such cases.
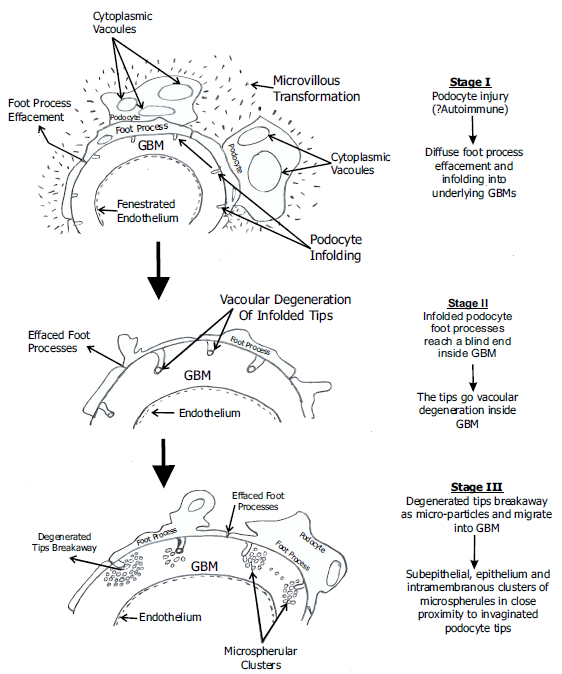
- Diagramatic representation of pathogenesis of PIG – Podocyte injury which is likely to be immune mediated -----> Infolding of podocyte cytoplasmic processes into underlying GBM ------> Degeneration of invaginated podocyte tips leads to ‘budding off’, either as microspherical/micro tubular particles or some as spheroid dense core particles. The former could be a result of vacoular degeneration of the tips which has been observed in a previously reported case from India.2 PIG - podocyte infolding glomerulopathy, GBM - glomerular basement membrane
The remodeled GBM in the later stages of MN could be more susceptible to invagination by the injured podocytes as it has been weakened by the intramembranous immune deposits. This could also account for the frequent co-occurrence of PIG with MN.1,2 The ultrastructural appearance of remodeled, irregular multi-lamellated GBM in Stage 4 MN closely resembles that of PIG, but the latter can be distinguished by identifying the infolded podocyte tips and surrounding clusters of microspherular/microtubular and dense core particles in PIG [Figures 2 and 3]. As the disease progresses, these particles are seen to gradually spread within the distorted and apparently weakened GBM. The origin of these particles from the infolded podocyte tips has been elegantly described in a previous study using immunoelectron microscopy and 3D reconstructed TEM images,7 and the characteristic ultrastructural appearance of PIG is well delineated today. Our case meets all the diagnostic ultrastructural features of PIG.
Our case showed strong positivity for PLA2R, with weak IgG positivity on IHC and absent MN-type immune deposits on EM. This incongruence in staining intensity, coupled with the absence of EM features of MN, prompts us to infer that PIG can occur as a PLA2R positive, primary/idiopathic entity, similar to primary MN. Most reported cases of PIG have been PLA2R negative and associated with other autoimmune diseases. This is much akin to the occurrence of idiopathic and secondary MN, lending credence to a common pathogenesis pathway.
Advances in proteomics have expanded the repertoire of target antigens for podocyte injury. Proteomic analysis of a sufficient number of cases would be required to accurately map the target podocyte antigens in PIG and unravel the pathogenesis of PIG.
Recently, whole exosome sequencing of a case series of PIG demonstrated mutations in INF2, one of the genes implicated in FSGS, raising the possibility of a genetic basis in the development of PIG.6 However, a direct causal relationship was not established and further studies are required in this direction.
Our patient responded well to immunosuppression, and his proteinuria has been in remission on follow-up for 2 years. This parallels the reported clinical course of PIG, wherein complete or partial remission has been attained with steroid monotherapy in most cases,4 with only a few cases requiring higher immunosuppressive regimens.5 Hence, it is important to make the distinction between PIG and MN for treatment purposes.
Conclusion
We recommend: 1. Routine EM evaluation in all cases diagnosed as MN on LM to identify more cases of PIG and distinguish PIG from MN. 2. Routine incorporation of immunohistochemical staining for PLA2R and other podocyte antigens alongside serum analysis for PLA2R in all cases of PIG. 3. Employing proteomics to pinpoint the target antigens on podocytes to further elucidate the pathogenesis of this rare and peculiar glomerulopathy.
Acknowledgements
The authors would like to thank Natalie Wilson, Advanced Biomedical Scientist, Tracey de Haro, Specialist Scientific Lead for Electron Microscopy, and the laboratory team at the University Hospital of Leicester for processing and sharing the images of electron microscopic analysis of this patient’s renal biopsy. The authors also thank the clinical immunology team at University Hospitals Birmingham and the laboratory team at University Hospital Coventry and Warwickshire for light microscopic evaluation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Proposal of podocyte infolding glomerulopathy as a new disease entity: a review of 25 cases from nationwide research in japan. Clin Exp Nephrol. 2008;12:421-31.
- [CrossRef] [PubMed] [Google Scholar]
- Podocyte infolding glomerulopathy (PIG) in a patient with undifferentiated connective tissue disease: a case report. Am J Kidney Dis. 2018;72:149-53.
- [CrossRef] [PubMed] [Google Scholar]
- Case Report: A peculiar glomerulopathy in a patient suffering from nephrotic syndrome. BMC Nephrol. 2019;22:326.
- [Google Scholar]
- Podocyte infolding glomerulopathy, first case report from north america. Can J Kidney Health Dis. 2021;8:20543581211048357.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Podocyte infolding glomerulopathy in a patient with phospholipase a2 receptor-positive membranous nephropathy and review of the literature. Nephron. 2021;145:496-502.
- [CrossRef] [PubMed] [Google Scholar]
- Podocyte infolding glomerulopathy: A case series report and literature review. J Clin Med. 2023;30:1088.
- [Google Scholar]
- Analysis of intra-GBM microstructures in a SLE case with glomerulopathy associated with podocytic infolding. Clin Exp Nephrol. 2008;12(6):432-439.
- [CrossRef] [PubMed] [Google Scholar]







