Translate this page into:
Poisoning and Envenomation Induced Acute Kidney Injury: A Hospital-Based Study
Corresponding author: Saif Quaiser, Department of Medicine, Jawaharlal Nehru Medical College, Aligarh Muslim University (AMU), Aligarh, India. E-mail: drssaif@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Khan A, Quaiser S, Khan R, Agrawal N. Poisoning and Envenomation Induced Acute Kidney Injury: A Hospital-Based Study. Indian J Nephrol. 2025;35:283-9. doi: 10.25259/IJN_3_2024
Abstract
Background
Most cases of acute kidney injury (AKI) in the Indian subcontinent are community-acquired. Some causes of AKI like poisonings are unique to the local demographics. This study examined the clinical features, spectrum, and outcomes of AKI in patients with poisoning and evaluated the predictors of mortality.
Materials and Methods
This was a prospective observational study conducted in patients admitted to Jawaharlal Nehru Medical College, Aligarh with an alleged history of poisoning orsnake bite. Relevant history, laboratory tests, mode of treatment, and outcome were recorded for all patients who were followed up after 3 months post discharge.
Results
During the study period, 394 patients were admitted with a provisional diagnosis of poisoning/snake bite analyzed, of whom 56 (14.2%) developed AKI. Final data analysis was done for 51 patients as 5 patients were lost to follow up. Paraquat poisoning was the most common cause of AKI, seen in 12 patients, followed by methanol in 9 patients. Hemodialysis was required in 29 (56.8%) patients. Complete recovery was seen in 33 (64.7%) patients, and 14 patients (27.5%) died during the acute phase of the illness. Late presentation to the hospital and presence of shock (mean arterial pressure <65 mmHg) on presentation were found to be associated with adverse outcomes (mortality/failure of return of renal function at 3 months). The most common cause of death was septic shock seen in 8 patients.
Conclusion
This study, which is probably the first from North India, highlights the fact that cases of poisoning/envenomation-related AKI contribute to a sizeable amount of morbidity/mortality.
Keywords
AKI
Dialysis
Copper sulphate
Poisoning
Snakebite
Introduction
Poisons are substances that, when absorbed, inhaled, or ingested in sufficient quantities, possess the potential to cause harm to organisms. A toxin is a poisonous substance produced within the living cells or organisms.1 Envenomation is the exposure to a toxin or a poison that results from a bite or sting from an animal such as a snake, scorpion, spider, or insect, or from marine life.2 As per the data from the National Crime Research Bureau (NCRB), a total of 1,64,033 suicidal deaths were reported in India in 2021.3 Ingestion of poison is one of the most common modes of suicide in low- and middle-income countries such as India.4 Among poisons, pesticides, and herbicides contribute to most cases of poisoning in India.5 Agricultural or household pesticides and drugs are taken intentionally, whereas intake of corrosives, kerosene, and other miscellaneous agents as well as animal bites happen accidentally.6,7 Identifying the patterns with the integration of preventive and promotive health services may help reduce morbidity and mortality associated with poisoning.8
Patients of poisoning are highly susceptible to kidney impairment; they often require hemodialysis and carry a high risk for mortality. In poisonings, acute kidney injury is known to affect prognosis in different manners. Kidneys function to eliminate the majority of toxicants, toxin clearance, therefore, is significantly impacted by acute kidney injury. Hemodialysis therapy is often used to enhance toxin removal.9
The existing literature on acute kidney injury (AKI) due to poisoning and envenomation is sparse, mainly comprising a few case reports and case series focussing on individual agents and their nephrotoxicity. Furthermore, no data are available on the incidence of poisoning-related AKI and its outcomes including risk for chronic kidney disease (CKD) and factors that can predict mortality. We describe the clinical profile, etiological agent, outcomes, need for hemodialysis, and predictors of mortality in patients with AKI due to poisoning seen at our center.
Materials and Methods
All patients >12 years of age presenting to the emergency of Jawaharlal Nehru Medical College, Aligarh between July 2021 and June 2023 with an alleged history of poisoning/snake or insect bite were enrolled in the study. Institutional ethical committee (IEC) approval was obtained before the commencement of the study. Informed consent was taken from all the participants. Patients with a prior history of CKD [estimated glomerular filtration rate (GFR) <60 mL/min/1.73 m2 or contracted kidneys on ultrasound examination] were excluded from the study.
Data collection
Detailed clinical history regarding the nature of the substance consumed, amount of poison consumed, the timing of consumption/bite, whether suicidal or homicidal, marital status, and any treatment or prior history of medical care before coming to our center, was noted. Vitals, level of consciousness, and urine output on presentation and during hospital stay were monitored. A diagnosis of snake bite was made from relevant clinical history along with the presence of characteristic fang marks on the bite site. Local examination of the bite site, whole blood clotting time, and repeated neurological assessment were done as per indication. The snake species could not be identified due to a lack of relevant history and technicalities.
Routine investigations like hemogram, renal function tests, liver function tests, ultrasound abdomen, chest x-ray, electrocardiogram (ECG), urine routine microscopy, viral markers, and arterial blood gas analysis were done in all patients on admission and repeated as and when needed. The Jaffe colorimetric method (Advia 1650 chemical analyzer, Bayer, Germany) was used to measure serum creatinine (Scr) concentration. Patients were classified as having acute kidney injury (AKI) based on the KDIGO 2012 definition.10
The Chronic Kidney Disease - Epidemiology Collaboration (CKD-EPI) creatinine equation (2009) was used to calculate the eGFR in our study.
Detoxification strategies
Gastric lavage was done in all patients with normal saline if no contra-indications were present. Specific antidotes as per institutional protocol including soda bicarbonate, folic acid, atropine, pralidoxime, methylene blue, penicillamine, zinc sulfate, and hydrocortisone were administered as per the indication. All snake bite patients were given 10 vials of polyvalent anti-snake venom on admission, and repeat doses were given if required 6 hourly.
RRT in the form of hemodialysis (HD)/sustained low-efficiency dialysis (SLED) using the Fresenius 4008S NG machine was given when indicated. The number of sessions of HD was noted per patient along with the duration of Intensive Care Unit/High Dependency Unit (ICU/HDU) stay and the need for any mechanical ventilation/inotropic support, if any.
The first follow-up visit was scheduled 2 weeks after discharge from the hospital and subsequent follow-up visits were scheduled every month for 3 months. Blood pressure, serum creatinine, and urine albumin excretion were checked at follow-up visits. The protocol was approved by our institutional ethics committee. Recovery was said to be complete if renal function returned to baseline within 3 months of exposure. No renal recovery was defined as mortality or failure of return of baseline renal function at 3 months after exposure.
Data organization and statistical analysis
Patients were grouped into AKI and non-AKI groups for analysis and renal function recovery and non-recovery groups for analysis. All categorical variables were expressed as frequencies and percentages, and continuous variables were expressed as mean ± 2 Standard Deviation (SD) with 95% confidence intervals or median with interquartile range (IQR) based on the normality of the data. Categorical variables between the two groups were compared by chi-square test. Continuous variables between two groups were compared by Student’s t-test or Mann–Whitney U test according to the distribution. Mortality between groups was expressed as relative risk (RR) with confidence intervals (CIs). A logistic regression analysis was done to assess the independent predictors of in-hospital mortality. A p-value <0.05 was taken as significant. The data was analyzed using the statistical software SPSS version 19.0 (IBM, Armonk, NY, USA).
Results
During the study period, 394 patients admitted with a provisional diagnosis of poisoning/snake/insect bite were analyzed, and 56 (14.2%) patients developed AKI. Final data analysis was done for 51 patients as 5 patients were lost to follow-up after discharge from the hospital.
Out of 51 patients with AKI 32 (62.7%) were males. 16 of the patients were addicted to alcohol, 7 were ganja smokers and 4 were addicted to both. 31 (60.8%) out of 51 cases had a suicidal intention of poison intake and 33 (64.7%) out of the 51 patients were married.
Compared with those in the non-AKI group, patients in the AKI group were older (46.8 ± 12.4 vs. 39.4 ± 15.1 years, p = 0.02) and had ≥1 comorbidities such as hypertension/diabetes/coronary heart disease (49.4% vs. 30.7%, p = 0.005) [Table 1]. The length of hospitalization was longer (9.7 ± 8.1 vs. 5.4 ± 3.1 days, p = 0.005), the time lapse between poisoning and hospitalization was significantly greater in the AKI vs. non-AKI group (32 ± 11.7 vs. 18 ± 5.8 hours, p < 0.001) and mean arterial pressure <65 mmHg upon admission was more frequent in the AKI group (40.1% vs. 10.8%, p < 0.001). Patients in the AKI group had lower serum bicarbonate levels (16.0 ± 5.0 vs. 20.0 ± 4.1 mmol/L, P < 0.001) upon admission. Notably, intensive care unit admissions (52.2% vs. 21.5%, p < 0.001), median highest dose of norepinephrine (IQR) 0.27 (0.12–0.44) vs. 0.12 (0.9–0.32) μg/kg/min (p < 0.001), and mechanical ventilatory support (36.6% vs. 8.9%, p < 0.001) were more frequently required in the AKI group. The mortality rate was higher in the AKI group than in the non-AKI group (27.45% vs. 1.1%, p < 0.001) as shown in Table 1. Four patients died in the non-AKI group (2 had ventricular tachycardia due to Celphos poisoning while 2 died of ventilator-associated pneumonia after organophosphorus poisoning).
| Characteristic | AKI (n = 51) | Non-AKI (n = 343) | p-value |
|---|---|---|---|
| Age (years) | 46.8 ± 12.4 years | 39.4 ± 15.1 years | 0.02 |
| Males | 32 (62.8%) | 214 (62.4%) | 0.767 |
| Comorbidities | 49.4 % | 30.7 % | 0.005 |
| Length of hospital stay (days) | 9.7 ± 8.1 days | 5.4 ± 3.1 days | 0.005 |
| Exposure admission time (hours) | 32 ± 11.7 hours | 18 ± 5.8 hours | <0.001 |
| MAP < 65 mmhg on admission | 40.1 % | 10.8 % | <0.001 |
| ICU admission | 22.2 % | 11.5 % | <0.001 |
| Mechanical ventilation | 19.1 % | 8.9 % | <0.001 |
| Serum bicarbonate (mmol/L) | 16.0 ± 5.0 mmol/L | 20.0 ± 4.1 mmol/L | 0.003 |
| Median highest dose of norepinephrine (IQR) — µ g/kg/min | 0.27 (0.12–0.44) | 0.14 (0.9–0.32) | <0.001 |
| Serum bilirubin (mg/dl) | 0.82 ± 0.67 mg/dL | 0.76 ± 0.55 mg/dL | 0.45 |
| Serum ALT (IU/L) | 59 ± 21.7 IU/L | 62 ± 20.1 IU/L | 0.37 |
| Hemoglobin (g/dL) | 11.2 ± 3.4 g/dL | 11.6 ± 4.1 g/dL | 0.33 |
| Total leucocyte count (mL) | 12.2 ± 4.7 × 103/mL | 11.6 ± 5.1 × 103/mL | 0.41 |
| Prothrombin time (seconds) | 16.3 ± 10.1sec | 17.0 ± 9.7 sec | 0.47 |
| Mortality rate | 14/51 (27.45%) | 4/343 (1.1%) | <0.001 |
µ: Mean, N: Numerical value, %: Percentage, AKI: Acute kidney injury, MAP: Mean arterial pressure, ICU: Intensive care unit, ALT: Alanine transaminase, IQR: Interquartile range.
A total of 45 patients had acute kidney injury (AKI) on admission [KDIGO AKI Stage 1–8 (14.2%), AKI stage 2–19 (33.9%) and AKI stage 3–12(26.6%)] and 6 (11.7%) patients developed AKI within 48 hours of admission (2 – AKI stage 2, 3 – AKI stage 3). KDIGO urine output and creatinine criteria were met in 33 (64.7%) patients, while 11 (21.6%) patients met only creatinine criteria and 7 (13.7%) met only urine output criteria. Eleven (78.5%) out of the 14 patients who died had AKI stage 3 on presentation as shown in Table 2.
| AKI KDIGO stage | No. of patients | Survival | Mortality | Relative risk (95%CI) |
|---|---|---|---|---|
| 1 | 15 | 14 | 1 | 0.29 (0.05–3.24) |
| 2 | 21 | 19 | 2 | 1.02 (0.22–6.56) |
| 3 | 15 | 4 | 11 | 4.15 (1.42–12.48) |
| Total | 51 | 37 (72.5%) | 14 (27.4%) |
AKI: Acute kidney injury, KDIGO: Kidney disease improving global outcomes, CI: Confidence interval.
Paraquat poisoning was the most common cause of acute kidney injury (AKI) seen in 12 patients, followed by methanol in 9, an unknown substance in 7, organophosphorus compounds in 5, snake bite in 5, and 2 cases each of copper sulfate, para-phenylenediamine, ammonium dichromate, wasp/bee sting poisoning. In 6 patients, multiple substances were ingested in combination. All the snake bite cases who developed AKI were vasculotoxic in nature with disseminated intravascular coagulation (DIC) developing in two of the patients.
RRT (SLED/HD using high flux dialyzer) was required in 29 (56.9%) of the patients out of which 22 had complete renal recovery (mean number of sessions 6.2 ± 2.3) as shown in Figure 1. All these seven patients who did not recover had AKI KDIGO stage III on presentation; 5 out of these succumbed during the hospital stay while 2 had increased serum creatinine even at 3 months of follow-up.
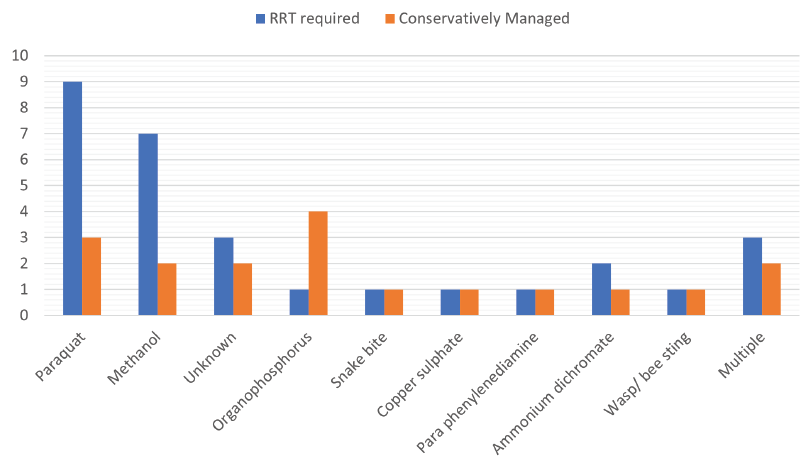
- Distribution of patients according to poisons and their dialysis requirement. RRT: Renal replacement therapy.
Complete recovery was seen in 33 (64.7%) patients, while 18 patients did not recover their renal functions at the end of 3 months of follow-up. Out of 18 patients who did not recover their renal functions, 14 (27.5%) died during the acute phase of illness as shown in Table 3.
| Poison | No. of patients | HD required | Survival | Mortality | P value |
|---|---|---|---|---|---|
| Paraquat | 12 | 9 | 5 | 7 | 0.007 |
| Methanol | 9 | 7 | 8 | 1 | 0.881 |
| Unknown | 7 | 3 | 6 | 1 | 0.244 |
| Organophosphorus | 5 | 1 | 4 | 1 | 0.596 |
| Snake bite | 5 | 1 | 5 | 0 | 0.596 |
| Copper sulphate | 2 | 1 | 2 | 0 | 0.865 |
| Para phenylenediamine | 2 | 1 | 1 | 1 | 0.08 |
| Ammonium dichromate | 2 | 2 | 1 | 1 | 0.08 |
| Wasp/Bee sting | 2 | 1 | 2 | 0 | 0.868 |
| Multiple | 5 | 3 | 3 | 2 | 0.11 |
| Total | 51 | 29 | 37 | 14 |
HD: Hemodialysis
Ten out of 14 patients who died, presented to the hospital more than 24 hours after poisoning/snakebite. Six patients died (11.8%) in the first 48 hours of admission, 5 (9.8%) died between 48 hours and 7 days, and 3 (5.9%) died in the second week of hospitalization. The most common cause of mortality was refractory septic shock seen in 8 patients while ventilator-associated pneumonia (acinetobacter baumannii related) was seen in 3 patients and one patient succumbed to snakebite-associated disseminated intravascular coagulation (DIC).
Renal biopsy was done in two out of the four patients with non-recovery of renal functions at the end of 4 weeks which showed acute cortical necrosis (ACN) in one patient (snake bite case) and acute tubular necrosis (ATN) (unknown poisoning) in one patient. The remaining two patients did not consent for biopsy. In five of the paraquat poisoning patients who had recovered their renal functions on follow-up, three patients developed diffuse alveolitis later on.
Paraquat exposure, late presentation to the hospital (after 24 hours), and the presence of shock (mean arterial pressure <65 mmHg) on presentation were the three variables found to be significantly associated with adverse outcomes in this study as evident in Table 4.
| Variable | Univariate analysis HR (95% CI) | P value | Multivariate analysis HR (95% CI) | P value |
|---|---|---|---|---|
| Age | 1.08 (0.76– 1.12) | 0.25 | ||
| Paraquat | 5.14 (2.67–12.38) | <0.001 | 4.88 (2.82-11.45) | <0.001 |
| Length of hospital stay | 1.34 (1.16–1.53) | 0.2 | ||
| ICU admission | 2.12 (1.68–4.72) | 0.006 | 1.89 (1.34-2.02) | 0.07 |
| Ionotropic requirement | 2.32 (1.21–4.12) | 0.005 | 1.35 (1.12-3.78) | 0.08 |
| Serum bicarbonate level | 1.06 (0.87–1.10) | 0.56 | ||
| AKI stage 3 | 4.58 (0.78–8.12) | 0.004 | 2.03 (0.79-4.15) | 0.09 |
| Exposure-admission time (hours) | 5.54 (3.12–11.67) | <0.001 | 5.26 (2.47-10.24) | <0.001 |
| MAP < 65 mm Hg on admission | 4.55 (2.46–7.92) | <0.001 | 4.16 (2.04- 7.01) | <0.001 |
| Mechanical ventilation | 2.34 (1.86–3.12) | 0.004 | 1.44 (1.08-2.78) | 0.04 |
| Comorbidities | 1.65 (1.44–2.32) | 0.13 |
HR: Hazard ratio, ICU: Intensive care unit, AKI: Acute kidney injury, MAP: Mean arterial pressure, CI: Confidence interval.
Discussion
According to WHO, approximately half of all deaths attributable to poisoning and envenomation worldwide are reported from India.11 Even though kidney injury is common, there is a lack of literature on AKI related to poisonings and envenomation, and its outcomes, and this study was a doorway to bridge this gap. The occurrence of AKI in patients of poisoning under study was 14.2%, considerably higher than the worldwide community-acquired AKI incidence of 8.3%.12
Compared with those in the non-AKI group, patients in the AKI group were older and had ≥1 comorbidities such as hypertension/diabetes/coronary heart disease. The time lapse between poisoning and hospitalization was significantly greater and the blood pressure was lower in the AKI vs. non-AKI group. The mortality rate was higher in the AKI group (18.3% vs. 1.0%, p < 0.001). The basic lab parameters including hemogram, liver function tests, and coagulation profile did not show any significant difference between the AKI and the non-AKI groups.
Data on renal recovery rate in poisoning-related AKIs is not available in the present literature. The figures, when compared to general rates of non-recovery in hemodialysis hemodialysis requiring AKI patients,13 were found to be marginally higher. As evident in our study, patients of poisoning either presenting or developing AKI KDIGO stage 3, carried a high risk of adverse outcomes including mortality and no return to baseline renal function after 3 when compared to earlier. The results possibly align with the initial management of sepsis, shock, and AKI, early initiation of hemodialysis, and delayed time of presentation to the hospital.
The highest mortality in poisoning-related AKI patients was seen during the acute phase of the illness. As established earlier also, the major cause of mortality was identified as sepsis in hospitalized AKI patients (41%);14 however, no such data is available specifically for poisoning-related AKI.
Paraquat poisoning was identified as the most common cause of AKI in our study. This non-selective herbicide has been widely used in agriculture since the 1960s.15 and poisoning due to this agent is known to carry high mortality. Studies have reported paraquat poisoning mortality rates ranging from 33.0% to 91.7%.16 The toxins build up in the lung tissue, causing the oxidation of lipids, the production of free radicals, and the depletion of nicotinamide adenine dinucleotide phosphate (NADPH). The primary cause of mortality in paraquat poisoning cases is lung involvement and acute alveolitis, later causing pulmonary fibrosis;17 however, in acute cases moderate-severe and fulminant poisoning cases, high mortality is attributable to acute renal injury, acute hepatitis, acute lung injury, and multiple organ failure.18,19 Despite the high requirement of hemodialysis, it is associated with grave outcomes20 which is consistent with the findings of the present study.
Methanol was the second most common agent observed in the poisoning AKI group. The prognosis was found to be better in comparison to the paraquat patients with 88.8% of patients showing recovery of their renal function. Methanol or methyl alcohol is an industrial substance, which upon ingestion is known to cause a wide variety of complications including cerebral hemorrhage in the putamen area, metabolic acidosis, visual impairment, hypokalemia, renal failure, respiratory failure, neurological failure, hypothermia, hypotension, low consciousness, and acute kidney injury (AKI).21 Amongst the list, AKI is one of the most common and related factors to mortality and morbidity.22,23 Several studies have shown a variable mortality rate, with as high mortality as 48%,24 but most of the studies date back to the 2000s.
Organophosphate (OP) poisoning was seen in nearly 10% with hemodialysis requirement in only one out of 5 patients. OP poisoning is common in developing countries like India, and carries an overall mortality of 4%–30%25 OPs are primarily known neurotoxins, but they also remain redoubted for their nephrotoxic effects with debatable and unclear mechanisms.26
In all the snake bite cases, snakes were identified to be vasculotoxic. Acute kidney injury (AKI) is a known life-threatening systemic effect of snake envenomation that commonly develops secondary to snake bites from the families of Viperidae and Elapidae.27 as per the previous studies based on the Indian population. AKI due to snake envenomation is usually severe and requires hemodialysis often, commonly causing ATN and AIN, with RCN being rare.27
Copper sulfate poisoning was seen in two patients. The substance is known to cause nephrotoxicity, however, the mechanism continues to remain unknown.28 Previous studies have reflected a high mortality associated with copper sulfate poisoning.29
Two patients presented with para-phenylenediamine poisoning, with 1 out of them requiring hemodialysis and failing recovery. Para-phenylenediamine (PPD), widely used in hair dye, characteristically is known to present with features of severe angioneurotic edema, intravascular hemolysis, and rhabdomyolysis and with hemoglobinuria culminating in acute kidney injury often requiring RRT.30 PPD poisoning, although rare in Western countries is not uncommon in low- and middle-income countries; and systemic toxicity is known to carry a high mortality rate (47.4%).31
An alleged history of wasp/bee sting was seen in 2 patients, both of them requiring hemodialysis, 1 of them showed recovery while the other succumbed during the acute phase of illness. The mechanism proposed behind AKI developing after a wasp or bee sting is thought to be ATN secondary to shock, AIN, direct venom nephrotoxicity, or pigment toxicity.32
Previous literature has concluded upon various parameters contributing to mortality in AKI including Stage 3 AKI, duration of AKI, length of hospital stay, and hyperkalemia.33 However, the studies included critically ill patients admitted to Intensive Care Units. No data on mortality assessment is available for AKI secondary to poisoning and envenomation. Paraquat exposure, late presentation to the hospital, and the presence of shock were the three variables found to be significantly associated with adverse outcomes in this study. The other variables studied did not show a statistically significant association with mortality in our study.
This study is the pilot exploration in the domain under study; and the results expose many more lacunas in the existing literature; thereby guiding many more large-scale multicentric studies to be done for further exploitation in the dimension of poisoning and envenomation-related kidney injury. The study also had a few limitations that included no monitoring of toxin serum levels, and the unavailability of details of the amount of poison ingested in most of the cases thereby limiting dose-effect relationship quantification. Continuous renal replacement therapy (CRRT) could have been invaluable in some of the patients who died and could not be dialyzed due to hemodynamic instability, its availability and affordability remain confined to a handful of government hospitals in our region. The present study being confined to a single center, does not provide external validity to all populations, considering the epidemiological variability in poisonings.
Patients presenting with poisoning and envenomation are quite common in our country, which is often complicated by the subsequent development of AKI. The highest risk of mortality occurs in the acute phase of illness, and aggressive management including detoxification strategies, volume resuscitation, and early initiation of hemodialysis as per indication are of paramount importance. Paraquat exposure, late presentation to the hospital (after 24 hours), and the presence of shock on presentation are significant predictors of mortality in poisoning cases in our cohort. Renal registries in this very overlooked cohort of patients with AKI can help us in better management of patients in the future.
Conflicts of interest
There are no conflicts of interest.
References
- Poisons and toxins. Science Learning Hub. Science Learning Hub; 2012. Available from: https://www.sciencelearn.org.nz/resources/364-poisons-and-toxins [Last accessed on 2023 December 29]
- Envenomation - an overview | ScienceDirect Topics. www.sciencedirect.com. Available from: https://www.sciencedirect.com/topics/medicine-and-dentistry/envenomation [Last accessed on 2023 December 29].
- Ministry of Health and Family Welfare, Government of India, National Crime Records Bureau. Available from: http://ncrb.gov.in/ [Last accessed on 2023 Dec 5th]
- Suicide key facts. Available: https://www.who.int/news-room/fact-sheets/detail/suicide [Last accessed on 2023 December 29]
- Suicide mortality in India: A nationally representative survey. Lancet. 2012;379:2343-51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute poisonings admitted to a tertiary level intensive care unit in northern India: Patient profile and outcomes. J Clin Diagn Res. 2015;9:UC01-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Experience of Indian emergency physicians in management of acute poisonings. Toxicol Commun. 2019;3:54-60.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Patterns and epidemiology of acute poisoning in ethiopia: Systematic review of observational studies. Arch Public Health. 2018;76:34.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care. 2004;8:R204-212.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138.
- [Google Scholar]
- Snakebite envenoming - A strategy for prevention and control. WHO Neglected tropical diseases/Snakebites reference number: 9789241515641, 2019c. Available: https://apps.who.int/iris/bitstream/handle/ 10665/324838/9789241515641-eng.pdf?ua=1 [Last accessed on 03 Dec 2023].
- Epidemiology and pathogenesis of acute kidney injury in the critically Ill patients. Indian J Crit Care Med. 2020;24:S84-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Renal recovery after acute kidney injury. Intensive Care Med. 2017;43:855-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Defining the cause of death in hospitalised patients with acute kidney injury. PLoS ONE. 2012;7:e48580. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3487783 [Last accessed on 03 Dec 2023]
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Failure of haemoperfusion and haemodialysis to prevent death in paraquat poisoning. Med Toxicol Adverse Drug Exp. 1988;3:64-71.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of early mortality in patients with paraquat intoxication. J Acute Med. 2013;3:6-10.
- [Google Scholar]
- Paraquat poisonings: Mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38:13-71.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features, treatment, prognosis, and mortality in paraquat poisonings: A hospital-based study in Iran. J Res Pharm Pract. 2019;8:129-36.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Paraquat-associated severe acute kidney injury—Study from India. J Ren Hepat Disord. 2022;6:14-23.
- [Google Scholar]
- Acute kidney injury in hospitalized patients with methanol intoxication: National inpatient sample 2003-2014. Hosp Pract. 2021;49:203-8.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for mortality in Asian Taiwanese patients with methanol poisoning. Ther Clin Risk Manag. 2014;10:61-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute kidney injury and the risk of mortality in patients with methanol intoxication. BMC Nephrol. 2019;20:205.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prognostic factors in methanol poisoning. Hum Exp Toxicol. 2007;26:583-6.
- [CrossRef] [PubMed] [Google Scholar]
- Study of organophosphorus compound poisoning in a tertiary care hospital and the role of peradeniya organophosphorus poisoning scale as a prognostic marker of the outcome. J Family Med Prim Care. 2021;10:4160-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A case report of acute kidney injury following organophosphate methidathion poisoning. Toxicologie Analytique et Clinique. 2022;34:121-6..
- [Google Scholar]
- Clinicopathological spectrum of snake bite-induced acute kidney injury from India. World J Nephrol. 2017;6:150-61.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Molecular insights of copper sulfate exposure-induced nephrotoxicity: Involvement of oxidative and endoplasmic reticulum stress pathways. Biomolecules. 2020;10:1010.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute renal failure following paraphenylene-diamine poisoning: A case report and review. Ren Fail. 2004;26:329-32.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical presentation, treatment and outcome of paraphenylene-diamine induced acute kidney injury following hair dye poisoning: A cohort study. Pan Afr Med J. 2014;19:163.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute kidney injury due to acute cortical necrosis following a single wasp sting. Ren Fail. 2013;35:170-2.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality and predictors of acute kidney injury in adults: A hospital-based prospective observational study. Sci Rep. 2021;11:15672.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







