Translate this page into:
Post Transplant Membranous Nephropathy: A 10-year Single-Centre Experience
Corresponding author: Sree Bhushan Raju, Department of Nephrology, Nizam’s Institute of Medical Sciences, Hyderabad, India. E-mail: sreebhushan@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gaggar P, Nair RR, Thakur AP, Raju SB. Post Transplant Membranous Nephropathy: A 10-year Single-Centre Experience. Indian J Nephrol. doi: 10.25259/IJN_150_2025
Abstract
Membranous nephropathy (MN) post-renal transplant can present as a recurrent or de novo disease, often impacting graft outcomes. We retrospectively analyzed 530 allograft biopsies over 10 years, identifying five MN cases (∼1%): four de novo (0.8%) and one recurrent. Among the former, 75% had concurrent antibody-mediated rejection (AMR); serum anti-PLA2R and tissue PLA2R were detected in 25%. All patients received plasmapheresis and low-dose intravenous immunoglobulin, with one requiring rituximab. Two patients stabilized, one experienced graft loss, and one attained complete remission. The recurrent MN case presented 10 years post-transplant and partially responded to rituximab. AMR influenced prognosis, with a 33% graft loss rate in de novo MN. Individualized treatment based on etiology, particularly targeting rejection, may improve outcomes. The study highlights the need for early diagnosis and personalized management in post-transplant MN.
Keywords
De novo
Membranous nephropathy
Post-transplant
Recurrent
Introduction
Membranous nephropathy (MN) is the leading cause of nephrotic syndrome in non-diabetic adults. In native kidneys, 80% of cases are primary (anti-PLA2R-mediated). Secondary MN arises from malignancies, infections, or autoimmune diseases.1 MN may be recurrent, de novo, or, rarely, donor-derived in allografts.2 We retrospectively analyzed post-transplant MN from January 2015 to December 2024, focusing on incidence, characteristics, and treatment response.
Case Series
Among 530 renal allograft biopsies over 10 years, MN was identified in 5 patients ( ̴1%). Four had de novo, and one had recurrent MN [Table 1]. Of the former, two received cadaveric transplants with anti-thymocyte globulin induction, and two had live donor transplants without induction. Chronic glomerulonephritis (n=1), membranoproliferative glomerulonephritis (n=1), IgA nephropathy (n=1), and Alport syndrome (n=1) were the underlying native kidney diseases.
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age/Sex | 71/M | 48/F | 32/M | 25/M | 27/F |
| Year of transplant | 2007 | 2017 | 2018 | 2019 | 2022 |
| Donor | Mother | Cadaver | Cadaver | Father | Father |
| Native kidney disease | MN | CGN | MPGN | IgAN | Alport syndrome |
| Induction IS | Nil | ATG | ATG | Nil | Nil |
| Time post-transplant to MN diagnosis (years) | 10 | 3 | 3 | 2 | 2 |
| Serum creatinine at presentation (mg/dL) | 1.6 | 3.27 | 4.3 | 1.4 | 0.95 |
| UPCR at presentation (g/g) | 3.8 | 1.1 | 4.2 | 5 | 2.6 |
| Serum albumin at presentation (g/dL) | 3.0 | 3.7 | 3.3 | 3.0 | 3.5 |
| Serum PLA2R (RU/mL) | NA | 20 | Negative | Negative | 62 |
| Hepatitis B or C | Negative | Negative | Negative | Negative | Negative |
| Concurrent antibody mediated rejection | Nil | Yes | Yes | Yes | No |
| Treatment given | Rituximab | PLEX + IVIG + Rituximab | PLEX + IVIG | PLEX + IVIG | Rituximab |
| Response to treatment | Attained partial remission | Serum creatinine -1.6 mg/dl with no decline in proteinuria | Serum creatinine – 2.64 mg/dl with decline in proteinuria | Serum creatinine – 1.0 mg/dl with decline in proteinuria | Attained complete remission |
| Follow-up duration (months) | 36 | 24 | 48 | 36 | 3 |
| Serum creatinine (mg/dL) at follow-up | 1.5 | 4.2 | 2.2 | 1.2 | 0.8 |
| UPCR (g/g) at follow-up | 0.5 | 2.86 | 0.7 | 0.6 | 0.2 |
| Outcome | Functioning graft | Died | Functioning graft | Functioning graft | Functioning graft |
CGN: Chronic glomerulonephritis, MPGN: Membranoproliferative glomerulonephritis, IgAN: IgA Nephropathy, IS: Immunosuppression, ATG: Antithymocyte Globulin, UPCR: Urine protein creatinine ratio, PLA2R: Phospholipase A2 receptor, NA: Not available, PLEX: Plasmapheresis, IVIG: Intravenous immunoglobulin, MN: Membranous nephropathy.
At diagnosis, the mean serum creatinine and UPCR were 2.48 ± 1.58 mg/dL and 3.23 ± 1.73 g/g, respectively, with a 2.5-year median onset post-transplant. Serum anti-PLA2R and tissue PLA2R were positive in 25% (1/4) of de novo cases. Renal histology revealed concurrent antibody-mediated rejection (AMR) in 75% (3/4) of cases [Supplementary Table S1]. All were given plasmapheresis and low-dose intravenous immunoglobulin, one also receiving rituximab. Renal function stabilized and proteinuria reduced in two patients, while one experienced progressive graft dysfunction and succumbed to infection in two years. The patient with Alport syndrome presented with sub-nephrotic proteinuria (2.6 g) and normal graft function; she received a single rituximab dose (500 mg) and achieved complete remission in three months. The recurrent MN case, PLA2R positive on biopsy, presented with nephrotic syndrome 10 years post-transplant and attained partial remission with two rituximab doses (500 mg).
Discussion
Four of 530 patients (0.8%) had de novo MN. Similarly, Aline-Fardin et al. reported an 18% (11/614) incidence over 17 years,3 while a Paris multicentre study reported 1.9% (19/1,000 biopsies).4 A recent Indian study analyzing post-transplant glomerulonephritis over 50 years reported no MN cases.5
MN’s recurrence rate in renal allografts varies between 10% and 50%,6,7 reflecting differences in clinical versus histological recurrence and for-cause vs. protocol biopsy use. Recurrence typically manifests within 1–3 years post-transplant,8 though our patient developed it 10 years post-transplant. This delayed onset may be attributed to reduced immunosuppression over time or an infection triggering auto-antibody resurgence.
Pathogenesis likely involves triggers like viral infection, urinary tract obstruction, ischemia, or rejection, exposed cryptic podocyte antigen, and in situ antigen-antibody complex formation, leading to a membranous glomerular injury pattern.S1 AMR’s histological features may coincide with or precede de novo MN.S2 All de novo MN cases (except the Alport syndrome case) were rejection-associated and PLA2R-negative. The literature on tissue PLA2R staining in de novo MN remains inconsistent. Larsen et al. reported almost always negative PLA2R staining, suggesting a distinct pathogenic mechanism in this form.S3 In contrast, de Sousa MV et al. found PLA2R-positivity in 75% of their cases.S4
A patient with autosomal recessive Alport syndrome, carrying a homozygous missense mutation in the COL4A4 gene, developed tissue and serum PLA2R-positive de novo MN [Figure 1] two years post-transplant and responded well to rituximab. Similarly, Prasad et al. reported a 10-year-old with Alport syndrome who developed de novo MN five years post-transplant.S5 We propose that alloimmune injury to the normal type IV collagen of transplanted kidney exposed cryptic PLA2R antigens on podocytes, triggering anti-PLA2R antibody formation and de novo MN.
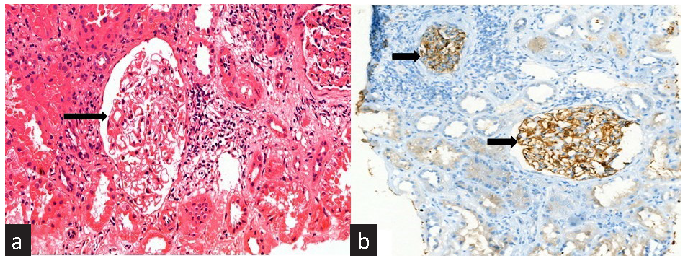
- (a) Light microscopy reveals diffuse thickening of the glomerular basement membrane (black arrow) (H&E stain, 400´ magnification). (b) Immunohistochemistry for PLA2R demonstrates diffuse granular positivity along the glomerular basement membrane (black arrows) (400´ magnification). PLA2R: Phospholipase A2 receptor.
De novo MN prognosis varies, primarily depending on the presence or absence of underlying rejection. A large French cohort found no significant impact of de novo MN on graft function,4 whereas Truong et al. reported allograft loss in 42% of cases, with a median time of three years post-diagnosis.S2 Treatment focuses on managing rejection, as seen in our series that observed a 33% graft loss rate.
Higher pre-transplant proteinuria and anti-PLA2R positivity increases recurrent MN risk. Grupper et al. found similar death-censored graft survival in MN and ADPKD patients, although 45% of recurrent MN cases experienced graft loss.7 Early rituximab therapy (>1 g/day proteinuria) may improve outcomes. Our recurrent MN patient achieved partial remission with rituximab, maintaining good graft function.
In summary, post-transplant MN had diverse etiologies, including recurrence, rejection-associated MN, and de novo PLA2R-positive MN. Individualized treatment based on the underlying cause, with a focus on achieving proteinuric remission, may improve graft outcomes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- The incidence of primary glomerulonephritis worldwide: A systematic review of the literature. Nephrology Dialysis Transplantation. 2011;26:414-30.
- [CrossRef] [Google Scholar]
- Membranous nephropathy transplanted in the donor kidney: Observations of resolving glomerulopathy in serial allograft biopsies. Nephrol Dial Transplant. 2014;29:2343-7.
- [CrossRef] [PubMed] [Google Scholar]
- Recurent and de novo membranous glomerulopathy after kidney transplantation. Transplant Proc. 2009;41:669-71.
- [CrossRef] [PubMed] [Google Scholar]
- Cooperative study of de novo extramembranous glomerulonephritis in renal allografts in humans: Report of 19 new cases in 1550 renal transplant patients of the transplantation group of the Ile de France. Nephrologie. 1982;3:158-66.
- [PubMed] [Google Scholar]
- Glomerulonephritis after renal transplatation in South Asia - single center experience over 5 decades. Indian J Nephrol. 2025;35:270-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Recurrent idiopathic membranous nephropathy after kidney transplantation: a surveillance biopsy study. Am J Transplantation. 2008;8:1318-22.
- [CrossRef] [Google Scholar]
- Recurrent membranous nephropathy after kidney transplantation: Treatment and long-term implications. Transplantation. 2016;100:2710-6.
- [CrossRef] [PubMed] [Google Scholar]
- De novo and recurrent membranous glomerulopathy following kidney transplantation. Transplantation. 1983;35:315-9.
- [CrossRef] [PubMed] [Google Scholar]







