Translate this page into:
Prevalence and predictors of chronic kidney disease in newly diagnosed human immunodeficiency virus patients in Owerri, Nigeria
Address for correspondence: Dr. I. I. Chukwuonye, Department of Internal Medicine, Federal Medical Centre, Aba Road, Umuahia, Abia State, Nigeria. E-mail: chukwuonye4@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Human immunodeficiency virus (HIV) infection is a common cause of chronic kidney disease (CKD) in Sub-Saharan Africa. This study aims at identifying the prevalence and predictors of CKD in newly diagnosed HIV patients in Owerri, South East Nigeria. This was a cross-sectional study consisting of 393 newly diagnosed HIV-seropositive subjects and 136 age- and sex-matched seronegative subjects as controls. CKD was defined as 24-hour urine protein (24-HUP) ≥0.3 g and/or glomerular filtration rate (GFR) < 60 ml/min. Subjects were recruited from the HIV clinic and the Medical Outpatient Department of Federal Medical Centre, Owerri. Clinical and anthropometric data were collected. Relevant investigations were performed, including HIV screening and relevant urine and blood investigations. The mean age of the HIV subjects was 38.84 ± 10.65 years. CKD was present in 86 (22.9%) HIV subjects and 11 (8.l %) controls. Low waist circumference (WC), high serum creatinine, high spot urine protein/creatinine ratio (SUPCR), high 24-HUP/creatinine Ratio (24-HUPCR), high 24-HUP/osmolality Ratio (24-HUPOR) predicted CKD in HIV subjects. CKD prevalence is high (22.9%) among newly diagnosed HIV patients in South East Nigeria. The predictors of CKD included WC, serum creatinine, SUPCR, 24-HUPCR, and 24-HUPOR.
Keywords
Chronic kidney disease
human immune virus
spot urine creatinine
spot urine osmolality
spot urine protein
Introduction
In 2013, about 35.3 million people worldwide were estimated to be living with human immunodeficiency virus (HIV).[1] Seventy percent of these live in sub-Saharan African countries.[1] In East African countries like Uganda, Kenya, and Tanzania, the prevalence of HIV-seropositive subjects is about 5%.[1] The prevalence in Central African countries like Cameroon and Gabon is about 5%.[1] In Nigeria, about 3.7% of the populations are HIV-seropositive.[1]
Human immunodeficiency virus-seropositive subjects are not only prone to chronic kidney disease (CKD) from HIV infection itself, but also from other causes of CKD that are observed in the general population as well as from nephrotoxic effects of some of the drugs that are used in treating HIV-seropositive subjects.
The global prevalence of CKD in HIV subjects has not been well-defined.[2] A large European study found a prevalence of 4%.[3] In USA, the prevalence ranges from 2% to 15.5%.[4567] Other studies showed similar prevalence in China Rwanda and Burundi.[8910]
There is dearth of data from South East Nigeria on prevalence of CKD in HIV subjects. In other parts of the country, some studies carried out showed that the prevalence of CKD among HIV patients ranges between 38.0% and 58.3%.[11121314]
Studies have also shown that predictors of CKD in HIV patients include Black race, increasing HIV ribonucleic acid level, absolute CD4 lymphocyte count less than 200 cells/ml, serum creatinine, presence of antibodies to hepatitis C virus, age >45 years and low estimated glomerular filtration rate (eGFR).[141516] A study in north-central Nigeria found CD4 cells count <200 cells/ml and older age in females as predictors of CKD in HIV patients.[17]
There are few studies on the prevalence and predictors of CKD in HIV subjects emanating from Nigeria; there is currently none from South East Nigeria. These findings prompted us to undertake this study in Federal Medical Centre, Owerri, south-east Nigeria.
Materials and Methods
This was a five-month cross-sectional study on the prevalence and predictors of CKD in newly diagnosed HIV patients. The survey was carried out between March 2011 and August 2011 in Federal Medical Centre, Owerri, Imo State, Nigeria. The study consisted of 393 newly diagnosed drug-naïve, HIV-seropositive adult subjects (in the age range of 18–65 years) and 136 age- and sex-matched HIV-seronegative subjects as control group. Exclusion criteria included subject below 18 years or above 65 years, febrile illnesses, evidence of heart failure, urinary tract infection, diabetes mellitus, hypertension, evidence of urological disease, and use of drugs that could interfere with urinary creatinine excretion. The same exclusion criteria applied to the control group. An interviewer-structured questionnaire was administered and relevant data were collected. These included patient's age, sex, diagnosis and co-morbidities (diabetic mellitus, hypertension, etc.).
Approval for this study was obtained from the Ethical and Research Committee of Federal Medical Centre, Owerri.
The biographic, anthropometric and other relevant data were captured with the aid of a questionnaire. Investigations performed by the subjects included HIV screening and confirmatory tests, spot urine protein (SUP), spot urine creatinine (SUCr), spot urine osmolality (SUOsm), serum creatinine (SCr), fasting serum lipid profile, hemoglobin (Hb), CD4 cells count, 24-h urine creatinine (24-HUCr), 24-h urine osmolality (24-HUOsm), 24-h urine protein (24-HUP),. Others include SUP/creatinine ratio (SUPCR), 24-HUP/creatinine ratio (24-HUPCR), 24-HUP/osmolality ratio (24-HUPOR), SUCr/osmolality ratio (SUCOR), 24-HUCr/osmolality ratio (24-HUCOR). CKD was defined as 24-HUP ≥0.300 g and/or GFR <60 ml/min.
Potential predictors of CKD evaluated were age, sex, serum creatinine, SUP, SUCr, SUOsm, 24-h urine volume (24-HUV), 24-HUCr, 24-HUOsm, SUPCR, SUPOR, 24-HUPCR, 24-HUPOR, SUCOR, 24-HUCOR, body mass index (BMI), waist circumference (WC), CD4 cells count, cholesterol, triglyceride (TG), low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), Hb, systolic blood pressure (SBP), and diastolic blood pressure (DBP).
There are no universally acceptable cut-off criteria for defining abdominal obesity using WC, given the existence of different criteria (including the International Diabetes Foundation,[18] and the Adult Treatment Panel III,[19] which are the more prominent criteria), and there are also different cut-offs for obesity for men and women. Bearing this in mind, we decided to use the Adult Treatment Panel III, which is commonly used in our country.
Statistical package for the social sciences (SPSS) version 17.0 (SPSS Inc., Chicago, IL, USA) statistical software was used to analyze the data. The strength of association between the variables and CKD was determined by correlation statistics while multivariate linear regression was used to quantify the association of variables to predict CKD. All tests were two-tailed with P < 0.05 taken as statistically significant.
Results
The mean age of the subjects was 38.84 ± 10.65 years, and their age ranged between 18 years and 65 years. The total number of the HIV seropositive subjects was 393 HIV; 283 (72.0%) were females while 110 (28.0%) were males. Eighteen subjects had incomplete investigation results and were removed from the study, making the actual number 375 subjects. The total number of the seronegative control subjects was 136 HIV; 98 (72.1%) were females while 38 (27.9%) were males. The female:male ratio was approximately 3:1 in both the HIV-seropositive subjects and the HIV-seronegative controls. The mean serum creatinine in HIV subjects was 1.03 ± 0.42 mg/dl, and in the control it was 0.88 ± 0.19 mg/dl (P < 0.001). The mean GFR (creatinine clearance) for the HIV subjects was 91.42 ± 22.98 ml/min, and in the control it was 93.01 ± 15.91 ml/min (P = 0.458).
Table 1 shows that out of the 375 HIV subjects, 86 (22.9%) had CKD compared with 11 (8.1%) of the 136 non-HIV controls. These were statistically significant.
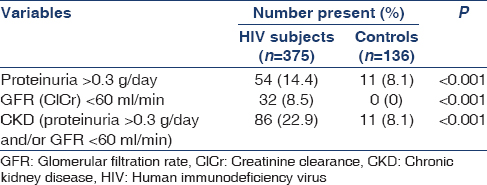
Table 2 shows the relationship between CKD and selected risk factors in the study population. The prevalence of CKD in HIV subjects among the different BMI categories was highest among those with BMI ≤18.5 (75%), and lowest among those with BMI ≥30 (28.75%). However, in the non-HIV control subjects the prevalence of CKD was highest among those with BMI of 18.5–24.9 (21.05%), while no case was seen in those with BMI of ≤18.5.
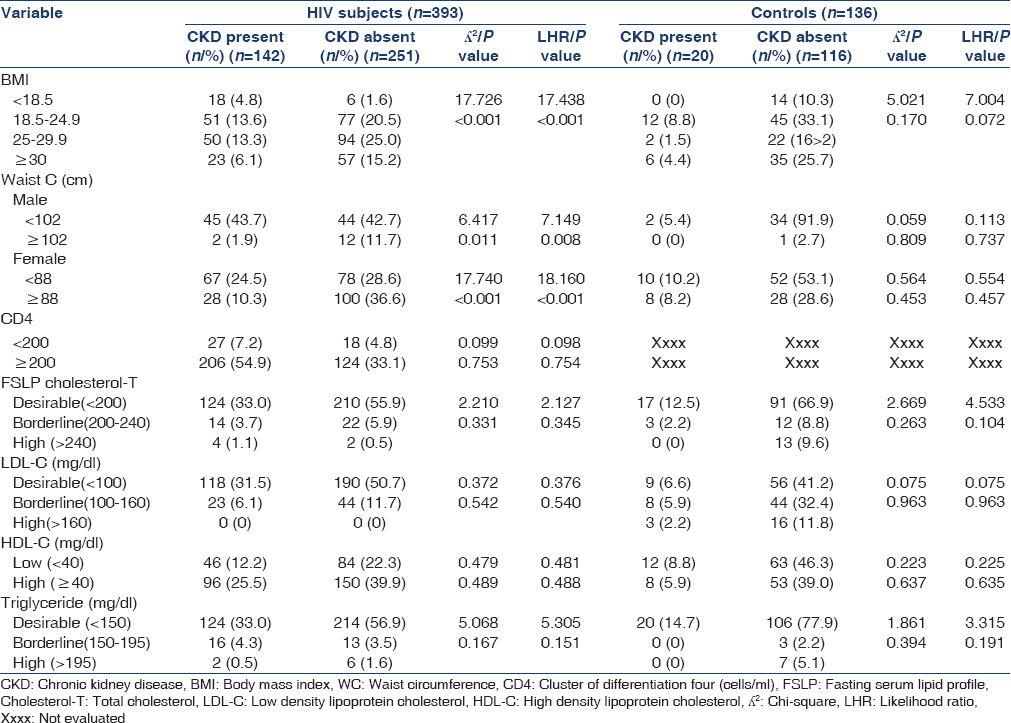
There was significant association between CKD in HIV subjects and WC in males (P = 0.011). A total of 89 males who were HIV seropositive had WC ≤102 cm. Of these, 45 (50.6%) had CKD. A total of 14 had WC >102 cm. Of these, only 2 (14.3%) had CKD. In addition, in males, prevalence of CKD in HIV increased as WC decreased. In females, a total of 145 had WC ≤88. Of these, 67 (46.2%) had CKD. A total of 128 had WC >88. Of these, 28 (21.8%) had CKD. In females, prevalence of CKD in HIV declined as WC increased.
There was no significant difference between the HIV subjects who had CKD and those without CKD in the different CD4 cell counts categories (CD4 <200 cells/mm3 P = 0.099 and CD4 ≥200 cells/mm3 P = 0.753). There were no statistically significant difference in lipid profile between HIV subjects with CKD and those without CKD, P = 0.331, LDL-C, P = 0.542, HDL-C, P = 0.489, TG, P = 0.167.
No significant association was found between CKD and the variables in the HIV-negative control group.
Table 3 shows correlation coefficient of CKD with BMI, WC, and Hb were −0.167, −0.297, and −0.142, respectively. P values were significant. The negative values indicate an inverse relationship. In contrast, the correlation coefficient of CKD with SCr, SUP, 24-HUV, SUPCR, SUPOR, 24-HUPCR, and 24-HUPOR were 0.123, 0.245, 0.235, 0.200, 0.256, 0.432, and 0.413, respectively. P values were significant. This implies a direct relationship between CKD and these factors.

There was no significant correlation between age, sex, SUCr, SUOsm, 24-HUCr, 24-HUOsm, SUCOR, 24-HUCOR, CD4 cells count, cholesterol, LDL-C, HDL-C, TG, SBP, and DBP with CKD.
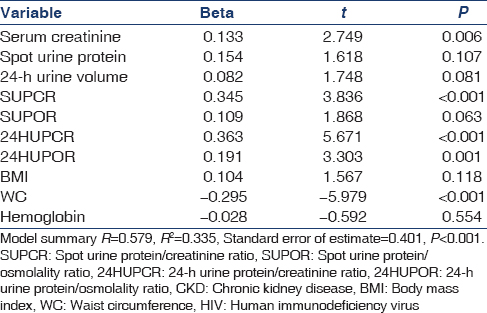
Table 4 shows a multivariate linear regression analysis of the potential predictors of CKD in HIV subjects. High SCr, high SUPCR, high 24-HUPCR, high 24-HUPOR, and low WC levels are predictors of CKD in the model. P ≤ 0.05 was taken as statistically significant.
Discussion
Our study showed that 22.9% of the HIV subjects had CKD, while 8.1% of the non-HIV controls had CKD. The study also showed that there was an inverse correlation between CKD and WC in HIV subjects. In addition, there was a direct correlation between CKD and SCr, SUPCR, 24-HUPCR, 24-HUPOR in HIV subjects. Multivariate linear regression showed that WC, SCr, SUPCR, 24-HUPCR, and 24-HUPOR were predictors of CKD in HIV subjects.
The CKD prevalence of 22.9% in HIV seropositive subjects is lower than the 53.3% prevalence observed by Okafor et al.[14] in Benin, south-south Nigeria. However, our finding is in keeping with some other studies carried out in other parts of Nigeria.[11121314]
In the study, low WC was found to be a predictor of CKD in male HIV subjects. This agrees with a study in which low BMI was found to be a predictor of CKD in HIV population.[11] We did not find any association between the female sex and CKD in HIV patients; this is in contrast to a study carried out in Northern Nigeria in which female gender was found to be predictive of CKD in HIV patients.[17]
The study showed that SUPCR, an index of 24-HUP, predicted CKD in HIV subjects. This is in keeping with a study in which proteinuria was found to be associated with the development of AIDS defining illness, progression to CKD, and an increased risk of death in HIV-infected patients.[20]
Both 24-HUPCR and 24-HUPOR were found to be predictors of CKD in HIV subjects in this study. In clinical practice the 24-HUPCR and 24HUPOR are not routine screening indices for CKD. However, there is dearth of studies that assessed the use of these parameters as potential predictors of CKD in HIV patients.
High serum creatinine was found to be predictive of CKD in HIV subjects in keeping with some earlier studies.[1116] However, the use of serum creatinine-based estimated GFR is more reliable in the assessment of renal function.[21]
Agbaji et al. showed in their study that there is an association between age and CKD in HIV patients. The study showed that older age was a predictor of CKD in HIV subjects. However, our study did not show such relationship, despite the fact that the mean age of the subjects in their study and ours was almost the same.
Our study did not find any association between cholesterol, TG, HDL, LDL, and CKD as was the case in similar earlier studies.[2223] However some earlier studies[67891011] showed that high total cholesterol and high TG predicted CKD and renal dysfunction, in HIV patients.
We did not find a significant association between CD4 cells count and CKD, unlike in some studies in which low CD4 cells count was found to predict CKD in HIV.[11242526] This observed difference could be adduced to the difference in study design.
Spot urine protein was associated with CKD in HIV patients in this study; however, the multivariate analysis showed that it is not a predictor of CKD in HIV subjects. The reason may be because semi-quantitative urine protein analysis or even quantitative SUP concentrations correlates poorly with the quantitative 24-HUP measurements due to variations in the levels of SUP during the day, as shown by a study.[27]
In this study, the results showed that low Hb was associated with CKD in HIV subjects; however, multivariate linear regression showed that Hb is not a predictor of CKD in HIV subjects. An earlier study had observed otherwise. This does not agree with the report of a study in which low Hb was found to be a predictor of CKD.[28]
The study also showed that there was no association between SBP, DBP, and CKD. In contrast, some studies reported SBP and DBP as predictors of CKD.[81122] This difference observed here may be explained by the exclusion of hypertensive patients in our study criteria and the high incidence of gastrointestinal conditions causing poor intake, volume depletion such as diarrhea and vomiting found in HIV patients. In addition, HIV patients are known to have some tubular dysfunction that may lead to salt-wasting nephropathy,[29] a condition that may possibly contribute to keeping the blood pressure low. However, this was not assessed in our study.
Conclusion
Chronic kidney disease prevalence is high (22.9%) among newly diagnosed HIV patients in south-east Nigeria. The predictors of CKD in HIV subjects include low WC (P < 0.001), high SCr (P = 0.006), high SUPCR (P < 0.001), high 24-HUPCR (P < 0.001), and high 24-HUPOR (P = 0.001) from our study.
The study reveals high prevalence of CKD in HIV subjects, and this brings to the fore, the need to investigate these subjects thoroughly in other to identify those patients at risk, and those that have renal disease. This will act as a guide in implementing potentially preventive and therapeutic strategies to whittle down CKD in HIV population.
Limitations of the Study
In this study, single assessment of urine protein and GFR was used for CKD instead of two assessments over a minimum of three months. This may have influenced the prevalence of CKD observed in the study. In addition, it was carried out in a single center in South East Nigeria. There is need for a multi-center study to give a thorough picture of the prevalence in south-east Nigeria.
Source of Support: Nil
Conflict of Interest: None declared.
References
- The changing epidemiology of HIV-related chronic kidney disease in the era of antiretroviral therapy. Kidney Int. 2014;86:259-65.
- [Google Scholar]
- Risk factors of chronic kidney disease in HIV-infected patients. Clin J Am Soc Nephrol. 2011;6:1700-7.
- [Google Scholar]
- Prevalence and factors associated with renal dysfunction among HIV-infected patients. AIDS Patient Care STDS. 2010;24:353-60.
- [Google Scholar]
- Factors associated with renal dysfunction within an urban HIV-infected cohort in the era of highly active antiretroviral therapy. HIV Med. 2009;10:343-50.
- [Google Scholar]
- Spectrum of chronic kidney disease in HIV-infected patients. HIV Med. 2009;10:329-36.
- [Google Scholar]
- Prevalence of chronic kidney disease in Chinese HIV-infected patients. Nephrol Dial Transplant. 2007;22:3186-90.
- [Google Scholar]
- Prevalence of kidney disease in HIV-infected and uninfected Rwandan women. PLoS One. 2011;6:e18352.
- [Google Scholar]
- Prevalence of chronic kidney disease among people living with HIV/AIDS in Burundi: A cross-sectional study. BMC Nephrol. 2011;12:40.
- [Google Scholar]
- Renal disease in HIV-seropositive patients in Nigeria: An assessment of prevalence, clinical features and risk factors. Nephrol Dial Transplant. 2008;23:741-6.
- [Google Scholar]
- Renal disease in the acquired immunodeficiency syndrome in north central Nigeria. Niger J Med. 2003;12:120-5.
- [Google Scholar]
- Prevalence of chronic kidney disease in HIV positive patients in Lagos, south-west Nigeria. Nephrology Rev. 2012;4:22-26.
- [Google Scholar]
- Renal disease in HIV infected patients at University of Benin Teaching Hospital in Nigeria. Afr Health Sci. 2011;11(Suppl 1):S28-33.
- [Google Scholar]
- Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis. 2004;39:1199-206.
- [Google Scholar]
- Prevalence of low estimated glomerular filtration rate, proteinuria, and associated risk factors among HIV-infected black patients using Cockroft-Gault and modification of diet in renal disease study equations. J Acquir Immune Defic Syndr. 2012;59:59-64.
- [Google Scholar]
- Predictors of impaired renal function among HIV infected patients commencing highly active antiretroviral therapy in Jos, Nigeria. Niger Med J. 2011;52:182-5.
- [Google Scholar]
- International Diabetes Federation Task Force on Epidemiology and prevention, Hational [sic] Heart, Lung, and Blood Institute, American Heart Association, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-5.
- [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-421.
- [Google Scholar]
- Development of proteinuria or elevated serum creatinine and mortality in HIV-infected women. J Acquir Immune Defic Syndr. 2003;32:203-9.
- [Google Scholar]
- Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830-8.
- [Google Scholar]
- Microalbuminuria and chronic kidney disease as risk factors for cardiovascular disease. Nephrol Dial Transplant. 2006;21:2366-74.
- [Google Scholar]
- HIV is associated with increased prevalence of microalbuminiria. Retroviruses Opportunistic Infect. 2005;12:821.
- [Google Scholar]
- Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int. 2002;61:195-202.
- [Google Scholar]
- Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis. 2004;39:1199-206.
- [Google Scholar]
- Quantitation of proteinuria with urinary protein/creatinine ratios and random testing with dipsticks in nephrotic children. J Pediatr. 1990;116:243-7.
- [Google Scholar]
- Severe renal dysfunction and risk factors associated with renal impairment in HIV-infected adults in Africa initiating antiretroviral therapy. Clin Infect Dis. 2008;46:1271-81.
- [Google Scholar]







