Translate this page into:
Prevalence of hypothyroidism in nondiabetic chronic kidney disease and effect of thyroxine replacement on estimated glomerular filtration rate
Address for correspondence: Dr. N. Purwar, Department of Medicine, M. L. N. Medical College and SRN Hospital, Allahabad, Uttar Pradesh, India. E-mail: naincypurwar@yahoo.co.in
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Reduced T3 and free T4, elevated thyroid stimulating hormone, and hyporesponsiveness to thyrotropin releasing hormone raise questions about the presence of hypothyroidism in chronic kidney disease (CKD) and raise the possibility of benefit from thyroxine supplementation. A prospective cohort study was conducted on 73 nondiabetic CKD cases. Hypothyroid patients were started on levothyroxine and were reviewed after 3 and 6 months. The mean age of study population was 42.3 ± 16.8 years. Of the total population, 32 (43.8%) cases had hypothyroidism, among whom 2 (2.7%) had overt hypothyroidism and 30 (41.1%) had subclinical hypothyroidism. Prevalence of hypothyroidism increased with increasing severity of CKD. There were 1 (3.1%) case with hypothyroidism in stage 3b, 8 (25%) cases in stage 4, and 23 (71.9%) cases in stage 5. The mean estimated glomerular filtration rate (ml/min/1.73 m2) at baseline was 13.7 ± 8.9 which increased to 17.5 ± 6.8 and 22.4 ± 9.3 after 3 and 6 months of thyroid hormone replacement therapy (THRT), respectively (P < 0.001). Hypothyroidism is commonly associated with nondiabetic CKD and its prevalence increases with declining renal function. THRT significantly improves renal function in nondiabetic CKD with hypothyroidism.
Keywords
Chronic kidney disease
estimated glomerular filtration rate
subclinical hypothyroidism
Introduction
Thyroid hormones have a significant impact on kidney disease so it is important to consider the physiological association of thyroid dysfunction in relation to chronic kidney disease (CKD). CKD affects the pituitary-thyroid axis and the peripheral metabolism of thyroid hormones.[1] Primary hypothyroidism is common in CKD patients. Especially, the prevalence of SCH increases consistently with a decline in estimated glomerular filtration rate (eGFR).[2]
The earliest and the commonest thyroid function abnormality in CKD patients is a “low T3 syndrome”. However, the free T4 levels vary from being low to normal, primarily because of impaired protein binding of T4. The thyroid profile is similar to that observed in several nonthyroidal illnesses (NTIs) such as severe infections, heart failure, malignancies, and in several hospitalized patients without renal disease. This led to the consideration of a “sick euthyroid state” in CKD, which is now called “nonthyroidal illness.” However, unlike other NTI states, there is no increase in total rT3 levels in CKD.[3] Another difference from other NTIs is that the thyroid-stimulating hormone (TSH) levels are elevated.
Thus, CKD patients have low T3 and normal or reduced T4 levels, and consequently elevated TSH and attendant increase in thyroid gland volume.[34] The reduced T3 levels and associated complications without an increase in rT3, the reduced free T4 levels along with an elevated TSH, and hyporesponsiveness of TSH to thyrotropin releasing hormone question the “euthyroid” state and raise the possibility of benefit from thyroid supplementation in CKD. There is still a lack of consensus in current guidelines on whether or not to treat SCH. In particular, little is known about the effect of thyroid hormone replacement on the changes in eGFR in CKD patients with SH. Only one previous study has examined the prevalence of hypothyroidism among patients with CKD not requiring dialysis. Among a small group of patients, 24% of study subjects had overt or subclinical hypothyroidism, with a higher prevalence among patients with diabetes.[5] A recent study on 113 CKD patients concluded that thyroid hormone replacement therapy (THRT) attenuated the rate of decline in renal function in CKD patients with subclinical hypothyroidism (SCH) suggesting that THRT may delay reaching end-stage renal disease in these patients.
Materials and Methods
With the permission of the Institutional Ethics Committee, a prospective cohort study was conducted on adult (age ≥18 years) CKD patients (confirmed as per kidney disease outcomes quality initiative guidelines) attending outpatient and inpatient department of our tertiary care center at Allahabad, India. Patients with known thyroid dysfunction or subjects who were receiving concurrent treatment with drugs that could contribute to hypothyroidism (lithium, amiodarone, or iodine) were excluded. Diabetic CKD patients were excluded as it may have an autoimmune predisposition for hypothyroidism. Demographic data were recorded, and a detailed history was taken from each patient including the history of hypertension, diabetes mellitus, and previous episodes of acute kidney injury and symptoms of uremia. Clinical examination and baseline investigations were done for enrolled patients, which included complete blood count, serum electrolytes, serum urea, and creatinine (simultaneously eGFR was calculated using MDRD* equation [GFR (mL/min/1.73 m2)] = 186 × [serum creatinine (mg/dl)]−1.154× (age [years])−0.203× (0.742 if female)).[6] Serum protein and albumin, thyroid function tests (free triiodothyronine [fT3], free thyroxine, TSH, and antithyroid peroxidase [TPO] antibody), serum intact parathyroid hormone, 24 h urine protein, and fasting and prandial blood sugars. Hypothyroidism was defined as a TSH level >4 mU/l. SCH was defined by a TSH >4 mU/l with normal fT3 level as per the European Thyroid Association guidelines 2013.[7] The normal reference range for TPO antibody was <0.5 IU/ml.[78] The American Diabetes Association criteria 2015 were used for diagnosis of diabetes mellitus.[9] All patients had an ultrasonography of abdomen for renal size, echogenecity, and corticomedullary differentiation.
A total of 73 nondiabetic CKD patients who were not known hypothyroids, were enrolled in the study and those with hypothyroidism, (qualifying the treatment recommendations)[7] were started on THRT with levothyroxine in a dose of 1.6 µg/kg body weight for overt hypothyroidism (OH) and 25–50 µg/day for SCH (lower doses for elderly patients). In patients with known cardiac illness the starting dose of levothyroxine was 12.5–25 µg/day. Reassessment of eGFR was carried out after 3 and 6 months. The dose of levothyroxine was adjusted on the basis of TSH levels, with the goal of treatment being normal TSH, preferably in the lower half of reference range. Data were analyzed using GraphPad Prism Software Inc. version 6.0 with the level of significance being 0.05. Numerical data were analyzed using paired and unpaired Student's t-test while z-test for proportions and Chi-square test was used for nonparametric data. Repeat measures ANOVA was used to evaluate difference in eGFR before and after THRT.
Results
Baseline characteristics of patients
The demographic, clinical, and biochemical data of the patients at the time of commencement of thyroid hormone (baseline) are listed in Table 1. The mean age of total study population was 42.3 ± 16.8 years and 53.4% of patients were male.

Prevalence of hypothyroidism in study population
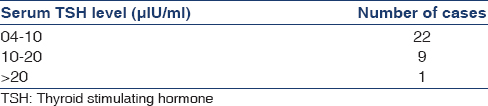
Among all cases, 32 (43.8%) were hypothyroid, 30 (41.1%) had SCH and 2 (2.7%) patients had OH. Prevalence of hypothyroidism increased with increasing severity of CKD. There were 1 (3.1%) case with hypothyroidism in stage 3b, 8 (25%) cases in stage 4 and 23 (71.9%) cases in stage 5. Table 2 shows the case distribution according to TSH levels.
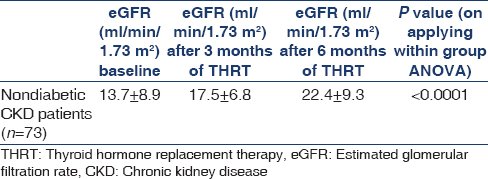
Comparison of estimated glomerular filtration rate before and after thyroid hormone replacement therapy
Table 3 shows the comparison of eGFR before and after THRT. The increment in eGFR was 3.8 ml/min/1.73 m2 and 8.7 ml/min/1.73 m2 after 3 and 6 months of THRT, respectively. The mean eGFR (ml/min/1.73 m2) at baseline was 13.7 ± 8.9 which increased to 17.5 ± 6.8 and 22.4 ± 9.3 after 3 and 6 months of THRT, respectively (P < 0.001).
Discussion
Even though previous studies have demonstrated that restoration of euthyroidism has beneficial effects on cardiac dysfunction in patients with SCH,[10] the impact of THRT on renal function has not been extensively explored in these patients. The results of this study show that thyroid hormone treatment significantly abrogated the decrease in eGFR in CKD patients with SCH and OH.
In this study, increased the prevalence of SCH and OH was found in nondiabetic CKD (41.1% subclinical and 2.7% overt). Previous studies also confirm that SCH is not a rare disorder in CKD patients.[21112] The data of 14,623 adult participants from the third National Health and Nutrition Examination Survey, a nationally representative sample of the United States population, revealed that the prevalence of hypothyroidism increased with lower levels of GFR, occurring in 10.9% of patients with stage 2 CKD, 21.0% with stage 3 CKD, and 23.1% with stage 4 or 5 CKD.[2] Moreover, Chonchol M et al.[13] showed that the prevalence of SCH increased from 7% at an eGFR 90 mL/min/1.73 m2 to 17.9% at an eGFR <60 mL/min/1.73 m2 in 3,089 unselected outpatient adults. Similarly in our study, the prevalence of hypothyroidism increased with declining levels of eGFR occurring in 3.1% of cases with stage 3b CKD, 25% cases with stage 4, and 71.9% of cases with stage 5 or end-stage renal disease. In the current study, we determined mean TSH levels across various stages of CKD to find a significant negative correlation (r = −0.07) between eGFR and TSH concentrations, which was in accordance with previous studies.[1415] The incidence of hypothyroidism is up to 4/1000 women and 1/1000 men and the prevalence of OH increases with age. SCH is found in 6–8% of women (10% over the age of 60yrs) and 3% of men.[16] This clearly demonstrates the higher prevalence of hypothyroidism in patients with chronic renal dysfunction.
The effect of thyroid hormone replacement on renal function has not been widely investigated in hypothyroid CKD patients, especially in SCH. A recent study by Shin et al.[5] demonstrated that thyroid hormone treatment not only preserved renal function but was also an independent predictor of renal outcome. However, they compared changes in eGFR in two different study populations. Thus, to clarify the direct impact of thyroid hormone treatment on the decline in renal function, it was imperative to compare decline in eGFR before and after L-thyroxine replacement in the same patient. The results of this study showed that THRT significantly improved the renal function as evidenced by mean eGFR (ml/min/1.73 m2) which increased from 13.7 ± 8.9 to 17.5 ± 6.8 and 22.4 ± 9.3 after 3 and 6 months of THRT, respectively (P < 0.001).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int. 2005;67:1047-52.
- [Google Scholar]
- Interactions between thyroid disorders and kidney disease. Indian J Endocrinol Metab. 2012;16:204-13.
- [Google Scholar]
- An evaluation of thyroid hormone status and oxidative stress in undialyzed chronic renal failure patients. Indian J Physiol Pharmacol. 2006;50:279-84.
- [Google Scholar]
- Thyroid hormone replacement therapy attenuates the decline of renal function in chronic kidney disease patients with subclinical hypothyroidism. Thyroid. 2013;23:654-61.
- [Google Scholar]
- A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-70.
- [Google Scholar]
- ETA guideline: Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-28.
- [Google Scholar]
- American Thyroid Association ATA/AACE Guidelines for Hypothyroidism in Adults, Endocr Pract. 2012;18(No. 6)
- American Diabetes Association. Standard of medical care in diabetes. Diabetes Care. 2015;38(Suppl 1):S1-2.
- [Google Scholar]
- Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549-55.
- [Google Scholar]
- The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76-131.
- [Google Scholar]
- Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1296-300.
- [Google Scholar]
- Association of thyroid function with estimated glomerular filtration rate in a population-based study: The HUNT study. Eur J Endocrinol. 2011;164:101-5.
- [Google Scholar]
- Correlation of creatinine with TSH levels in overt hypothyroidism – A requirement for monitoring of renal function in hypothyroid patients? Clin Biochem. 2012;45:212-4.
- [Google Scholar]
- Harrison's principles of internal medicine Vol Vol. 341. (18th ed). McGraw-Hill Companies; 2015. p. :2918-9.







