Translate this page into:
Profile, Risk Factors, and Outcomes of Asymptomatic Bacteriuria in Kidney Transplant Recipients with Normal Pretransplant Genitourinary Tract: A Single-Center Experience
-
Received: ,
Accepted: ,
How to cite this article: Mani SS, Thomas A, Alam R, Lalwani M, Valson AT, Yadav B, et al. Profile, Risk Factors, and Outcomes of Asymptomatic Bacteriuria in Kidney Transplant Recipients with Normal Pretransplant Genitourinary Tract: A Single-Center Experience. Indian J Nephrol 2024;34:37-44. doi: 10.4103/ijn.ijn_407_22
Abstract
Introduction:
There is a paucity of studies on asymptomatic bacteriuria (ASB) among kidney transplant recipients (KTR) in developing countries. This study assessed the clinical profile, risk factors, outcomes, and impact of treatment of ASB in KTRs with a normal genitourinary tract.
Methods:
Consecutive KTRs from 2009 to 2018 with no clinical or radiological evidence of obstructive uropathy were included. Urinary tract infection (UTI) after ASB was defined as occurrence of cystitis, pyelonephritis, or urosepsis, with ASB being the first bacteriuric episode.
Results:
Seven hundred ten out of 794 patients with median follow up of 47 months were included. The mean age was 35.5 ± 12 years. Eighty-one patients (11.4%) developed ASB at a median of 25 days (IQR 10, 134.5). Fifty-three percent and 4.9% of ASB episodes were extended-spectrum beta-lactamase (ESBL) positive and carbapenem-resistant organisms, respectively. Eighteen patients (32.1%) with early ASB (<3 months) and 5 (20%) with late ASB developed UTI on follow-up. Fifty-five percent of early and 16% of late ASB episodes were treated, with no significant difference observed in the risk of development of UTI when compared to untreated ASB episodes.
Conclusion:
The incidence of ASB as first bacteriuric episode in our cohort was 11.4%, with there being significant antimicrobial resistance. Female gender, pretransplant UTI, and delayed graft function were independently associated with development of ASB. Treatment of ASB episodes either early or late did not decrease the risk of development of UTI.
Keywords
Asymptomatic bacteriuria
kidney transplant
normal pretransplant genitourinary tract
urinary tract infection
Introduction
Infectious complications remain the major cause of morbidity and mortality among kidney transplant recipients (KTRs) in the developing world. Urinary tract infection (UTI) is the most common bacterial infection reported after transplant surgery,1,2. Asymptomatic bacteriuria (ASB) is especially common in the first year after transplant, with an incidence varying from 4% to 51% depending on the definition used.3 During the early post-transplant period, ASB is often treated due to high level of immunosuppression and proximate use of indwelling bladder catheter and/or ureteral stent. In recent years, a number of randomized control trials4–6 have been conducted on the benefit of treating ASB more than two months after kidney transplantation.7 None of these trials have come from a developing country to support or oppose this approach. In developing countries, community-acquired UTIs have shown increasing rates of extended-spectrum beta-lactamase (ESBL) organisms.8–10 Screening and treatment of ASB involves concern over the selection of resistant strains and the financial burden, especially in resource-limited settings.5 Abnormal pretransplant urinary tract, a significant risk factor for progression to UTI,11 has not been excluded in most of the studies. In this study, we looked at the profile, risk factors, and outcomes of ASB in KTRs, with special reference to its treatment and drug-resistant organisms.
Materials and Methods
Study design and population
Consecutive KTRs who underwent kidney transplant from 2009 to 2018 at Christian Medical College, Vellore and having a normal pretransplant genitourinary tract were included. A normal pretransplant genitourinary tract was defined by the absence of lower urinary tract symptoms with no clinical or radiological evidence of abnormal lower urinary tract and no recurrent urinary tract infections. This study protocol was approved by the institutional review board and ethics committee (IRB no. 12265/2020). Clinical, demographic, and laboratory data were collected from the medical records.
Kidney transplant protocol
Induction therapy consisted of either IL-2 receptor antagonist basiliximab or anti-thymocyte globulin based on the immunological risk. Maintenance immunosuppression included prednisolone, a calcineurin inhibitor and an anti-metabolite. All patients received valganciclovir and trimethoprim-sulfamethoxazole for three and six months, respectively. Pretransplant urine cultures were sterile. KTRs were given a single intravenous dose of 1.2 g of amoxicillin-clavulanate and 1 g of ceftazidime as preoperative antibiotic prophylaxis, as per the transplant unit protocol. Double J (DJ) stent was placed in all deceased donor transplants. However, in live donor–related transplants with normal pretransplant genitourinary tract, the stent was placed if there were any intraoperative complications. Foley’s catheter was removed on day 5 and DJ stent was removed on day 14 after the transplant. Urine culture using standard techniques was done seven days after the transplant, monthly for four months, and then every three months till completion of one year and thereafter at every post-transplant follow-up. The decision on treating ASB was as per the choice of the treating physician. The duration of treatment for ASB, cystitis, acute graft pyelonephritis/urosepsis were five, seven and fourteen days respectively.
Definitions
ASB and UTI were defined as per the guidelines from the Infectious Diseases Community of Practice (IDCOP) of the American Society of Transplantation.7 ASB was defined as more than 105 bacterial colony-forming units per milliliter (significant growth) of a uropathogen in the urine without symptoms. Patients in whom a second successive urine culture (voided or suprapubic aspirate) was done but who did not show the presence of significant growth of uropathogen were not considered to have ASB. Early ASB was defined as the first bacteriuric ASB episode occurring less than three months after transplant. UTI event after an ASB episode was classified as cystitis, acute graft pyelonephritis, or urosepsis. Cystitis (lower-tract UTI) was defined as the significant growth of a uropathogen in the urine culture with dysuria, frequency, or urgency but no systemic symptoms and no in-dwelling device. Acute graft pyelonephritis was defined as the significant growth of a uropathogen in the urine and at least one of the following symptoms: fever, chills, malaise, pain over the allograft, or graft dysfunction. Urosepsis was defined as the significant growth of a uropathogen in the urine with features of graft pyelonephritis and bacteremia of the same organism as grown in the urine. Patients with ASB as the first bacteriuric episode were divided into Groups A and B. Group A included patients who developed a UTI event after an initial ASB episode, whereas Group B included those who did not develop UTI events during the subsequent follow-up.
Statistical analysis
Baseline demographic data were presented as mean (standard deviation, SD) and median (interquartile range, IQR) for continuous variables and as numbers and percentages for categorical variables. The characteristics of patients in Group A (UTI event after ASB) and in Group B (-No UTI events during the follow-up) were compared using a t test for normally distributed continuous data and using the Mann–Whitney U test for non-normally distributed continuous data. The categorical data were compared using the Chi-squared test or Fisher’s exact test, as appropriate. Kaplan–Meir survival curves and logrank test was used to estimate the outcomes of ASB. The hazard ratios of risk factors of developing ASB and their 95% confidence intervals were derived from a univariate Cox model, with P values corresponding to the Wald test. Statistical significance was defined as P < 0.05. All analyses were performed using the IBM SPSS Statistics version 25 and R software.
Results
Seven hundred ninety-four patients underwent kidney transplant during the study period [Figure 1]. Out of them, 710 (77.5% males, mean age 35.6 years, 10.6% diabetic) met the inclusion criteria [Table 1]. Basiliximab was the most common induction agent (71.7%). Prednisolone, mycophenolate, and tacrolimus were the maintenance immunosuppressive agents administered in 94.2% of patients. Three hundred eighty-six bacteriuric episodes occurred in 181 patients (25.5%), with a median follow-up of 47 months (IQR 27, 77). The first episode of bacteriuria occurred at a median of 25 days (IQR 10, 134.5) after transplant. Eighty-one out of 181 patients had ASB and the rest had UTI as the first bacteriuric episode.
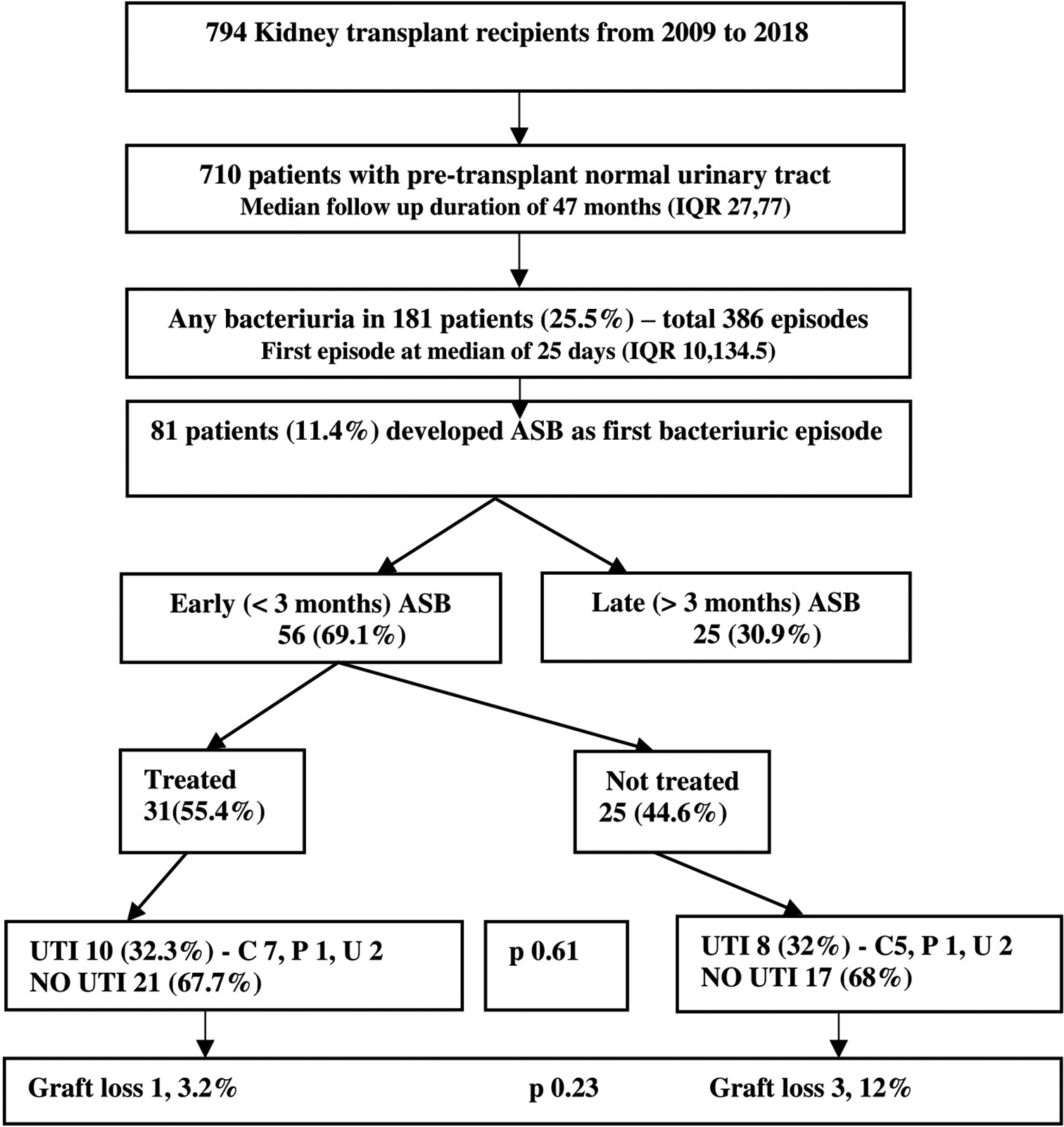
- Outcome of patients with early ASB on follow-up. All values are denoted as N (%). ASB = Asymptomatic bacteriuria, UTI = Urinary tract infection, C = Cystitis, P = Pyelonephritis, U = Urosepsis.
| Variables (n [%] or mean±SD) | Asymptomatic bacteriuria | Unadjusted analysis | Adjusted analysis | |||
|---|---|---|---|---|---|---|
| Yes (n=81) | No# (n=529) | 95% CI | P | 95% CI | P | |
| Age in years | 37.6±13.2 | 35.2±11.2 | −0.67–5.48 | 0.12 | ||
| Female gender | 37 (45.7) | 96 (18.1) | 2.32–6.19 | 0.00 | 2.39–6.55 | 0.00 |
| Body mass index (kg/m2) | 21.6±3.8 | 21.1±3.7 | 0.3–1.44 | 0.20 | ||
| Mode of dialysis: | ||||||
| Preemptive | 12 (14.8) | 57 (10.8) | −4.89–1.6 | 0.69 | ||
| HD | 64 (79) | 451 (85.2) | ||||
| PD | 5 (6.2) | 21 (4) | ||||
| Dialysis vintage in months* | 7 (3.5, 11.5) | 7 (3, 11) | 0.61 | |||
| ABO incompatible transplant | 1 (1.2) | 13 (2.5) | 0.06–3.84 | 0.50 | ||
| Comorbidities | ||||||
| Diabetes mellitus | 12 (14.8) | 50 (9.5) | 0.84–3.28 | 0.14 | ||
| Hypertension | 77 (95.1) | 481 (90.9) | 0.18–1.48 | 0.22 | ||
| Pretransplant UTI episodes | 7 (8.6) | 16 (3) | 1.20–7.62 | 0.018 | 1.2–6.88 | 0.015 |
| HBV infection | 4 (4.9) | 18 (3.4) | 0.48–4.47 | 0.49 | ||
| HCV infection | 2 (2.5) | 26 (4.9) | 0.11–2.10 | 0.33 | ||
| Native kidney disease: | ||||||
| Glomerular | 20 (24.7) | 164 (31) | ||||
| Unknown | 22 (27.2) | 148 (28) | 0.85–1.15 | 0.92 | ||
| Diabetic | 18 (22.2) | 80 (15.1) | ||||
| Interstitial | 15 (18.5) | 72 (13.6) | ||||
| Others | 6 (7.4) | 65 (12.3) | ||||
| Transplant characteristics: | ||||||
| Donor age in years | 43.4 (10.8) | 42.1 (11.3) | −1.39–4.06 | 0.33 | ||
| Deceased donor | 11 (13.6) | 50 (9.5) | 0.74–3.02 | 0.25 | ||
| Female Donor | 44 (54.3) | 34 (64.5) | 0.44–1.19 | 0.21 | ||
| ATG as induction agent | 26 (32.1) | 131 (24.8) | 0.86–2.38 | 0.16 | ||
| Maintenance immunosuppression: | ||||||
| TAC + MMF | 80 (98.8) | 494 (93.4) | 0.03–1.54 | 0.12 | ||
| CSA + MMF | 1 (1.2) | 29 (5.5) | ||||
| TAC + AZA | - | 1 (0.2) | ||||
| Others | - | 5 (0.9) | ||||
| Delayed graft function | 14 (17.3) | 31 (5.9) | 1.69–6.63 | 0.00 | 1.99–8.35 | 0.00 |
| CMV infection after transplant | 19 (23.5) | 84 (15.9) | 0.92–2.85 | 0.09 | ||
| New onset DM after transplant | 20 (24.7) | 132 (25) | 0.57–1.69 | 0.96 | ||
ASB: Asymptomatic bacteriuria, HD: Hemodialysis, PD: Peritoneal dialysis, UTI: Urinary tract infection, HBV: Hepatitis B virus, HCV: Hepatitis C virus, ATG: Anti-thymocyte globulin, TAC: Tacrolimus, MMF: Mycophenolate mofetil, CSA: Cyclosporine, AZA: Azathioprine, CMV: Cytomegalovirus, DM: Diabetes mellitus, *median (IQR), #patients without any episode of ASB or UTI
Spectrum of asymptomatic bacteriuria and outcome
Eighty-one patients (11.4%) developed ASB as the first bacteriuric episode. The most common organism isolated was Escherichia coli (49.4%) followed by Klebsiella (17.3%). Fifty-three point one percent of those isolates were ESBL-producing strains and 4.9% were carbapenem-resistant [Table 2]. Female gender, history of UTI before kidney transplant, and delayed graft function were independently associated with the development of ASB [Table 1].
| Organisms | n=81 | % | Median (IQR) time to ASB (days) | Median WBC/HPF in urine |
|---|---|---|---|---|
| Escherichia coli | 40 | 49.4 | 23 (19, 389) | 5 (1, 33) |
| Klebsiella | 14 | 17.3 | 19 (9, 58) | 0 (0, 58) |
| Enterococcus | 11 | 13.6 | 20 (7, 771) | 3 (0.75, 16) |
| Pseudomonas | 5 | 6.2 | 26 (17, 96) | 12 (0, 12) |
| Others | 11 | 13.5 | 31 (17, 719) | 2 (2, 68) |
| Resistance patterns | ||||
| ESBL-positive | 43 | 53.1 | 19 (9, 36) | 3 (0, 17) |
| CRO | 4 | 4.9 | 18 (9, 24) | 5 (4, 5) |
| Pan susceptible | 13 | 16 | 498 (28, 2130) | 3 (0.8, 83) |
| Others | 21 | 25.9 | 36 (15, 745) | 2 (0, 17) |
CRO: Carbapenem resistant organisms, ESBL: Extended-spectrum beta-lactamase, HPF: High power field, WBC: White blood cell
On follow-up, 23 of 81 patients (28.4%) developed a UTI event at a median of 63 days. Risk factors for development of any UTI event after an ASB episode were studied. None of the pre- or post-transplant factors included in the study were statistically significant [Table 3]. There was no statistical difference between the two groups with respect to death censored graft survival, all-cause mortality, or the estimated glomerular filtration rate (eGFR) at five years using the CKD-EPI creatinine equation.
| Variables n (%) | Group A, n=23 (n, %) | Group B, n=58 (n, %) | 95% CI (%) | P |
|---|---|---|---|---|
| RISK FACTORS | ||||
| Pretransplant factors | ||||
| Age >50 years | 5 (21.7) | 12 (20.7) | 0.35–2.59 | 0.94 |
| Pretransplant UTI | 2 (8.7) | 5 (8.7) | 0.25–4.71 | 0.89 |
| Pretransplant DM | 4 (17.4) | 8 (13.8) | 0.26–2.28 | 0.77 |
| Pretransplant HBV | 2 (8.7) | 2 (3.4) | 0.43–7.86 | 0.44 |
| Female recipient | 12 (52.2) | 25 (43.1) | 0.33–1.73 | 0.76 |
| Glomerular NKD | 6 (26.1) | 14 (24.1) | 0.37–2.40 | 0.91 |
| Pretransplant DSA | 2 (8.7) | 3 (5.2) | 0.32–5.91 | 0.65 |
| Deceased donor | 3 (13) | 8 (13.8) | 0.33–3.80 | 0.84 |
| ATG induction | 10 (43.5) | 16 (27.6) | 0.89–4.67 | 0.08 |
| Female donor | 14 (66.7) | 30 (56.6) | 0.29–1.81 | 0.50 |
| DJ stenting | 11 (47.8) | 18 (31) | 0.22–1.15 | 0.10 |
| Post-transplant factors | ||||
| Delayed graft function | 3 (13) | 11 (19) | 0.40–4.63 | 0.60 |
| Foleys catheter removal >5 days | 14 (60.9) | 25 (43.1) | 0.78–4.22 | 0.16 |
| PTDM | 8 (34.8) | 12 (20.7) | 0.24–1.35 | 0.20 |
| Any rejection | 7 (30.4) | 11 (19) | 0.23–1.39 | 0.21 |
| CMV infection | 4 (17.4) | 10 (17.2) | 0.32–2.84 | 0.95 |
| ESBL-positive organisms | 14 (60.9) | 33 (56.9) | 0.55–2.95 | 0.56 |
| Pyuria (>5 WBC/HPF) | 11 (57.9) | 15 (38.5) | 0.77–4.76 | 0.15 |
| Time to ASB<30 days | 7 (30.4) | 28 (48.3) | 0.20–1.19 | 0.11 |
| OUTCOMES | ||||
| CKD-EPI eGFR at 1 year | 71.9±21.7 | 76.4±20.9 | −15.5–6.8 | 0.44 |
| CKD-EPI eGFR at 5 years | 76.5±23.4 | 73.3±25.4 | −17.2–23.6 | 0.75 |
| Graft loss | 4 (17.4) | 3 (5.2) | 0.06–1.22 | 0.09 |
| All-cause mortality | 5 (27.1) | 3 (5.2) | 0.89–16.71 | 0.07 |
Group A: UTI event after ASB, Group B: No UTI on subsequent follow up, ASB: Asymptomatic bacteriuria, ATG: Anti-thymocyte globulin, CKD-EPI eGFR: Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate, CMV: Cytomegalovirus, DJ: Double J, DM: Diabetes mellitus, DSA: Donor specific antibody, HBV: Hepatitis B virus, HPF: High power field, NKD: Native kidney disease, PTDM: Post transplant diabetes mellitus, UTI: Urinary tract infection, WBC: White blood cell
Effect of timing, drug resistance, and treatment on outcomes of ASB
The following subgroup analyses were done Based on the first bacteriuric episode, ASB episodes were divided into early and late ASB. Among the 56 patients with early ASB, 31 (55.4%) were treated [Figure 1]. However, among the 25 patients with late ASB, only 4 (16%) were treated [Figure 2]. MDR organisms were more common in patients with early ASB episode than in those with late ASB episode (69.6% vs. 32%, 95% CI = 1.76–13.45; P = 0.003). However, the death censored graft survival did not differ (P = 0.83) between early ASB episodes (estimate = 110.2 months, 95% CI = 100.2–120.2) and late ASB episodes (estimate = 118.2, 95% CI = 105.3–131.1). There was no statistically significant difference (P = 0.34) in death censored graft survival between MDR ASB organisms (estimate = 101.3 months, 95% CI = 83.9–118.5) and non-MDR ASB organisms (estimate = 120.7 months, 95% CI = 109.8–131.7).
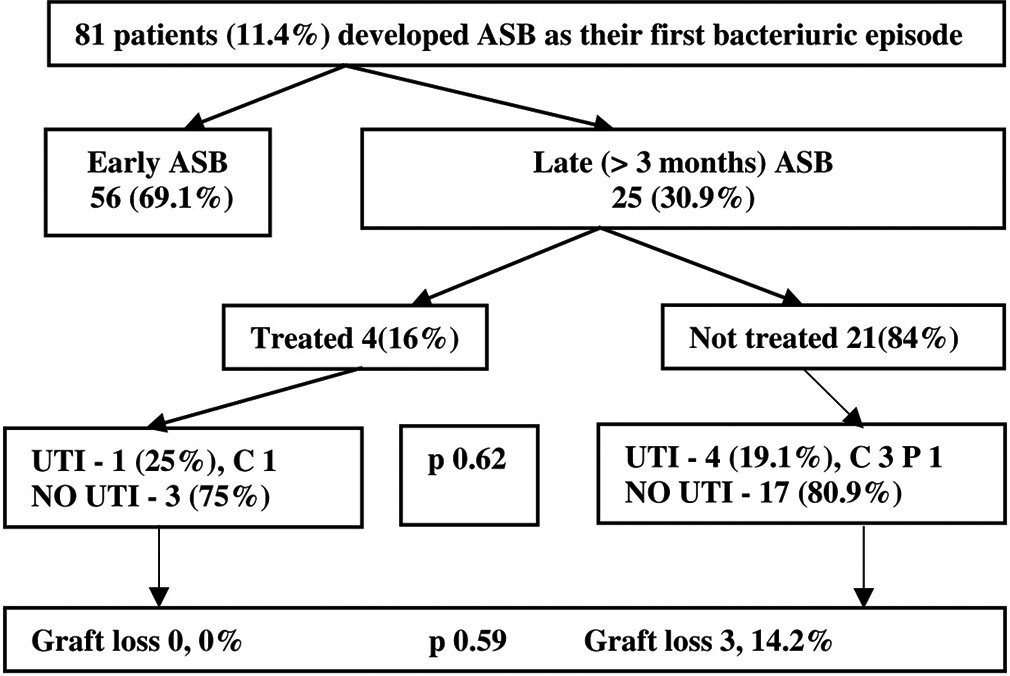
- Outcome of patients with late ASB on follow-up. All values are denoted as N (%). ASB = Asymptomatic bacteriuria, UTI = Urinary tract infection, C = Cystitis, P = Pyelonephritis.
Treatment of ASB neither prevented a UTI event on follow-up (estimate = 86.3 months, 95% CI = 73.3–99.3 vs. estimate = 100.1 months, 95% CI = 84.9–115.3; P = 0.35) nor resulted in better graft survival (estimate = 107.1 months, 95% CI = 101.7–112.6 vs. estimate = 109.5 months, 95% CI = 95.5–123.5; P = 0.083) when compared to untreated ASB episodes. Figure 3 shows the time trend of treating ASB by year. The average cost of treating an episode of ASB was 160 USD. There was no difference in rate of resistant organisms isolated in subsequent urine cultures in the patients whose ASB were treated when compared to those with untreated ASB (54.3% vs. 43.5%; P = 0.375). Among patients who had untreated ASB episodes with MDR organisms, 68% of them spontaneously cleared the organisms on subsequent screening, which was similar to pan susceptible organisms (81%).

- Time trend of treating ASB over the years. All values are denoted as N. ASB = Asymptomatic bacteriuria.
Discussion
Screening and treatment of ASB is based on the risk of it progressing to graft pyelonephritis and subsequently affecting graft survival.12,13 Overall, the incidence of ASB reported in literature—varying due to time period of screening, duration of follow-up, frequency of testing, and geographical location—is 4%–51%.4,14–17 The low prevalence of ASB (3.4%) reported by Coussement et al.3 is probably because of the exclusion of early ASB episodes (first two months). A study by Sharma et al.18 with a smaller sample size and shorter duration of follow-up reported the incidence of ASB to be 41.79%. Table 43-6,14-26 summarizes the previously published studies on ASB in KTRs. Observational cohort studies done in a developing country like India are minimal.
| Country, year | Study design | Total patients | ASB n (%) | Treated n (%) | Time from transplant | Other results |
|---|---|---|---|---|---|---|
| Iran, 200519 | Single center RCT | 88 | 88 | 43 (48.8) | 12 months | The number of ASB episodes and symptomatic UTIs did not differ significantly between the treated and untreated groups (P >0.05). |
| Spain, 201014 | Retrospective | 189 | 96 (50.8) | 96 (100) | 0–36 months | No differences in graft function at 36 months between patients with no ASB and those screened and treated for ASB |
| Switzerland, 201120 | Retrospective | 196 | 77 (39.2) | 101 (30) | More than 1 month | Persistent ASB in 45 (46%) treated episodes. Selection of resistant pathogen in 35 patients (78%). Spontaneous clearance in 138 (59%) untreated ASB. |
| Israel, 201315 | Retrospective | 656 | 112 (17) | 22 (19.6) | 1–12 months | Resistant bacteriuria in 36% of treated patients. No benefit for treatment in the short- and long-term follow-up |
| Poland, 201416 | Retrospective | 209 | 83 (38) | 83 (100) | 0–12 months | ASB was an independent risk factor for symptomatic UTIs, but only 21 of 152 episodes of symptomatic UTIs were preceded by ASB with the same causative agent. |
| Spain, 201621 | Cross sectional | 538 | 48 (8.9) | 28 (58.3) | Not specified | Untreated ASB – no hospitalization, 70% spontaneous bacterial clearance. 47.6% of patients with treated ASB showed new resistance to another antibiotic. |
| Spain, 20164 | Single center RCT | 112 | 112 | 53 (47.2) | 2–24 months | Systematic screening and treatment of ASB beyond the second month after transplantation provided no apparent benefit. |
| Netherlands, 201617 | Retrospective | 343 | 63 (18.4) | NA | 0–6 months | TMP-SMX as Pneumocystis jiroveci prophylaxis was not associated with reduced prevalence of ASB. |
| Singapore, 201722 | Retrospective | 171 | 41 (24) | 41 (100) | More than 1 month | MDR organisms accounted for 43.9% of infections. Female sex and deceased donor recipients were independent predictors of 30-day bacteriuria. One-year patient and graft survival were similar in recipients with or without ASB. |
| Australia, 201723 | Retrospective | 276 | 75 (27) | 139/324 episodes | 0–12 months | Untreated ASB followed by symptomatic UTI was significantly higher when compared to those who were treated. |
| USA, 201924 | Retrospective | 527 | 64 (12) | 48 (74.6) | 0–12 months | Treatment not protective against ASB-to-UTI progression even in the first month post-transplant. |
| India, 201918 | Prospective | 67 | 28 (41.8) | NA | 0–6 months | ASB was more common in deceased donor transplant and those with growth in ureteral stent culture. There was no compromise in allograft function at 6 months. |
| France, 201925 | Retrospective | 77 | 37 (48) | 7 (18.9) | 2–24 months | Multidrug-resistant bacteria in 27% of the patients. No benefit in systematic treatment of ASB in pediatric KTR |
| Europe, 20193 | Cross-sectional | 500 | 17 (3.4) | More than 2 months | Prevalence of ASB was low. ASB significantly associated with female gender and older age. | |
| Spain, 20195 | Multicenter RCT | 205 | 87 (42.4) | 41 (47.1) | Less than 12 months | There were no differences in the occurrence of pyelonephritis, urosepsis, cystitis, acute rejection, graft loss, and mortality between the treated and untreated groups. Antibiotic resistance was increased in the treatment arm. |
| Belgium/France, 20216 | Multicenter RCT | 199 | 199 | 100 (50.2) | More than 2 months | A screen-and-treat strategy for ASB did not reduce the occurrence of UTI in kidney transplant recipients for whom it had been more than 2 months since transplantation. Furthermore, this strategy increases antibiotic use and promotes the emergence of resistant organisms. |
| Spain, 202126 | Prospective | - | 175 | 54 (30.8) | Anytime | At six months, 6 (11.1%) treated versus 4 (3.3%) untreated ASB patients had UTI episodes (P=0.07, OR 3.65, 95% CI = 0.98–13.53). |
| Our study | Observational | 710 | 81 (11.4) | 35 (43.2) | Anytime | 58% of ASB episodes were caused by MDR organisms. Treating early or late ASB episodes did not result in decreased risk of development of UTI. |
ASB: Asymptomatic bacteriuria, RCT: Randomised control trial, TMP-SMX: Trimethoprim Sulfamethoxazole, UTI: Urinary tract infection
Risk factors for development of ASB include female gender, chronic glomerulonephritis, pretransplant diabetes mellitus, Double J stenting, older age, and second transplant.3,14,16,20 In our cohort, female gender, history of UTI before kidney transplant, and delayed graft function were independently associated with ASB. E. coli and Klebsiella were the common pathogens causing ASB in our study, as reported in the literature.3,15,20
Significant antimicrobial resistance (42.2% ESBL-positive and 3.9% carbapenem-resistant) was noted among our community-acquired uropathogens too.9 Similar to a study from Poland two-third of ASB episodes presented early, which indicates that most of the ASB are hospital-acquired as the pre-transplant urine cultures were sterile.16 Despite MDR organisms being isolated mainly in the early ASB episodes, death censored graft survival did not differ between early and late ASB episodes.
A UTI event after an ASB episode, reported from a Cleveland clinic, was similar to our cohort (28.4%).24 Jayanth et al.,27 from our center, reported increased rates of pyelonephritis and epididymo-orchitis in patients with pretransplant abnormal lower urinary tract. Hence, those patients were excluded from our study. Pre- and post-transplant risk factors did not predict the development of UTI after an ASB episode [Table 3]. The role of bacterial functional virulence factors and metabolism in the development of UTI after an ASB episode has not been assessed in our study.28 Even the presence of pyuria in urinalysis led to inappropriate treatment and serious consequences, and it did not predict the risk of progression to UTI.29 UTI event after an ASB episode did not affect the graft and patient survival in our cohort, as reported in the literature.14,18 However, it might be worth looking at the outcomes of ASB with MDR organisms prospectively.
Current consensus or guidelines on treatment of early ASB episodes and its impact on patient and graft outcomes are limited. In this cohort, nearly half of the early ASB episodes were treated but did not show any difference in development of UTI events or decline in delta eGFR at five years. Bohn et al.24 reported that ASB episodes were treated in 74.6% of the patients, with there being no added benefit. Unwarranted treatment of ASB episodes may lead to increased risk of MDR organisms and increased economic burden in developing countries.30,31 Kotagiri et al.23 reported that treatment of ASB led to statistically lesser episodes of UTI when compared to those untreated. However, this was a retrospective study with no standardized protocol for treatment of ASB. Green et al.15 reported that treatment of ASB led to a higher risk of hospitalization for UTI or more than 25% reduction in eGFR. Reported randomized control trials did not show any benefit with a screen-and-treat strategy for ASB two months after transplantation.4–6 Origüen et al.4 reported no benefits in treating ASB more than two months after kidney transplantation. However, only half of the patients assigned to the treatment group strictly fulfilled the planned treatment protocol for every episode of ASB. Sabé et al.5 too reported no added benefit in the treatment of ASB with added risk of antibiotic resistance. Published guidelines and studies on ASB do not provide clear guidance on management of early ASB episodes. The merits of our study are larger sample size and longer follow-up (median follow-up of 47 months); its limitation was its retrospective design. The study also highlights the profile, outcomes, and impact of treating the first ASB episode in KTRs with a normal pretransplant genitourinary tract from predominant live related donors. This contrasts with the published literature which included predominant deceased donors. The benefits of treating an early ASB could be studied from a multicenter randomized control trial with an adequately powered sample size.
Conclusion
ASB is common among KTRs in the first three months since the transplant . E. coli was the most common pathogen, with significant ESBL-positive or carbapenem-resistant organisms detected. The screen-and-treat strategy can be avoided as there is no additional benefit in graft survival or reduction in UTI events. With the current literature available, the screening for ASB could be limited to the first three months and treatment can be given only if there is progression to a UTI event. The financial implications of screening for ASB and the emergence of antibiotic resistance add to the burden on the healthcare system in a resource-limited setting.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Infectious complications after kidney transplantation: Current epidemiology and associated risk factors. Clin Transplant. 2006;20:401-9.
- [CrossRef] [PubMed] [Google Scholar]
- Common infections in kidney transplant recipients. Clin J Am Soc Nephrol. 2012;7:2058-70.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of asymptomatic bacteriuria among kidney transplant recipients beyond two months post-transplant: A multicenter, prospective, cross-sectional study. PloS One. 2019;14:e0221820.
- [CrossRef] [PubMed] [Google Scholar]
- Should asymptomatic bacteriuria be systematically treated in kidney transplant recipients? Results from a randomized controlled trial. Am J Transplant. 2016;16:2943-53.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic treatment versus no treatment for asymptomatic bacteriuria in kidney transplant recipients: A Multicenter randomized trial. Open Forum Infect Dis. 2019;6:ofz243. doi: 10.1093/ofid/ofz243
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotics versus no therapy in kidney transplant recipients with asymptomatic bacteriuria (BiRT): A pragmatic, multicentre, randomized, controlled trial. Clin Microbiol Infect. 2021;27:398-405.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary tract infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13507.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding the patterns of antibiotic susceptibility of bacteria causing urinary tract infection in West Bengal, India. Front Microbiol. 2014;5:463.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-microbiological profile of urinary tract infection in south India. Indian J Nephrol. 2011;21:30-6.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic resistance in pathogens causing community-acquired urinary tract infections in India: A multicenter study. J Infect Dev Ctries. 2008;2:354-8.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary tract infections in kidney transplant recipients: Role of gender, urologic abnormalities, and antimicrobial prophylaxis. Ann Transplant. 2013;18:195-204.
- [CrossRef] [PubMed] [Google Scholar]
- How early postoperative urinary tract infections affect renal graft function at 1-year follow-up. Transplant Proc. 2020;52:2403-8.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary tract infections in kidney transplant recipients-Is there a need for antibiotic stewardship? J Clin Med. 2022;11:226.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic screening and treatment of asymptomatic bacteriuria in renal transplant recipients. Kidney Int. 2010;78:774-81.
- [CrossRef] [PubMed] [Google Scholar]
- Consequences of treated versus untreated asymptomatic bacteriuria in the first year following kidney transplantation: Retrospective observational study. Eur J Clin Microbiol Infect Dis. 2013;32:127-31.
- [CrossRef] [PubMed] [Google Scholar]
- Treated asymptomatic bacteriuria during first year after renal transplantation. Transpl Infect Dis. 2014;16:605-15.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of trimethoprim-sulfamethoxazole as Pneumocystis jiroveci pneumonia prophylaxis on the occurrence of asymptomatic bacteriuria and urinary tract infections among renal allograft recipients: A retrospective before-after study. BMC Infect Dis. 2016;16:90.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum of asymptomatic bacteriuria in renal allograft recipients and its short-term effect on graft outcome: Experience of a Tertiary Care Center from Northwest India. Indian J Transplant. 2019;13:20.
- [CrossRef] [Google Scholar]
- Effect of antibiotic therapy on asymptomatic bacteriuria in kidney transplant recipients. Urol J. 2005;2:32-5.
- [Google Scholar]
- Outcome of treated and untreated asymptomatic bacteriuria in renal transplant recipients. Nephrol Dial Transplant. 2011;26:4109-14.
- [CrossRef] [PubMed] [Google Scholar]
- Short-term outcome of untreated versus treated asymptomatic bacteriuria in renal transplant patients. Transplant Proc. 2016;48:2941-3.
- [CrossRef] [PubMed] [Google Scholar]
- Screening for asymptomatic bacteruria at one month after adult kidney transplantation: Clinical factors and implications. Clin Transplant. 2017;31:e12954.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary tract infections in the first year post-kidney transplantation: Potential benefits of treating asymptomatic bacteriuria. Transplant Proc. 2017;49:2070-5.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of asymptomatic bacteriuria incidence and management post-kidney transplantation. Clin Transplant. 2019;33:e13583.
- [CrossRef] [PubMed] [Google Scholar]
- Asymptomatic bacteriuria in pediatric kidney transplant recipients: To treat or not to treat? A retrospective study. Pediatr Nephrol. 2019;34:1141-5.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of treating asymptomatic bacteriuria in kidney transplant recipients: A prospective cohort study. Antibiotics (Basel). 2021;10:218.
- [CrossRef] [PubMed] [Google Scholar]
- Renal transplantation into optimized abnormal lower urinary tract-Impact on graft outcomes, patient survival, and complications. Indian J Urol. 2019;35:67-72.
- [CrossRef] [PubMed] [Google Scholar]
- Asymptomatic bacteriuria in kidney transplant recipients-A narrative review. Medicina (Kaunas). 2023;59:198.
- [CrossRef] [PubMed] [Google Scholar]
- Deconstructing the urinalysis: A novel approach to diagnostic and antimicrobial stewardship. Antimicrob Steward Healthc Epidemiol ASHE. 2021;1:e6.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic selective pressure and development of bacterial resistance detected in bacteriuria following kidney transplantation. Transplant Proc. 2015;47:1131-5.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for infection with extended-spectrum and AmpC ß-lactamase-producing gram-negative rods in renal transplantation. Am J Transplant. 2008;8:1000-5.
- [CrossRef] [PubMed] [Google Scholar]







