Translate this page into:
Radiation-Induced Renal Artery Stenosis
Corresponding author: Kosha Patel, Department of Nephrology, Muljibhai Patel Urological Hospital, Nadiad, Gujarat, India. E-mail: kosha1012d@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Patel K, Gang S, Hegde U, Konnur A, Patel H. Radiation-Induced Renal Artery Stenosis. Indian J Nephrol. 2025;35:296-8. doi: 10.25259/ijn_485_23
Abstract
We present a case of a 33 year old male presenting with uncontrolled hypertension, requiring four drugs and renal dysfunction. Investigations revealed calcific atherosclerosis of abdominal aorta and bilateral renal artery stenosis. He had received radiotherapy for abdominal Non-Hodgkin’s lymphoma 14 years ago. Transluminal angioplasty was done on the right side resulting in improved renal function and normalization of blood pressure. Radiotherapy for intra-abdominal malignancy induces atherosclerosis process in arteries within field of irradiation and they manifest after many years of the initial insult.
Keywords
Accelerated atherosclerosis
Hypertension
Percutaneous angioplasty
Radiation
Renal artery stenosis
Renal dysfunction
Introduction
Radiation therapy, either alone or with chemotherapy and surgery is a part of most protocols for cancer treatment. The literature describes the incidence of accelerated atherosclerosis post radiation to be upto 40%.1 We report a case of radiation induced renal artery stenosis (RAS) presenting 14 years after the treatment.
Case Report
A 33-year old male presented to our department with complaints of uncontrolled hypertension and renal dysfunction. His past medical records showed that in 2002 he noticed multiple painless swelling over medial aspects of thighs, left calf and axillary region, skin nodules over anterior abdominal wall and weight loss. Abdominal ultrasound showed multiple lymph nodes. Bone marrow was uninvolved. The thigh lesion was biopsied, diagnosed as stage IV B lymphoblastic lymphoma and received chemotherapy with MCP-842 regimen and four cycles of MCP-841 for the same. Involved field radiotherapy (IFRT) to the bilateral thigh and abdomen, pelvis was given for a soft tissue lobulated mass 2.4 cm × 2.2 cm × 2.1 cm in the splenorenal region anterior to the left kidney with a total dose of 4500 Gy in 25 fractions over 37 days. No recurrence on follow up. Renal function and blood pressure monitoring on OPD visits were normal till 2020, when he was diagnosed to have a blood pressure of 190/100 mmHg. His blood pressure was poorly controlled despite the addition of multiple anti-hypertensives. In October 2022, he presented with complaints of headaches, nausea, and blood pressure of 200/110 mmHg. Urine examination showed trace proteinuria with normal sediments. His serum creatinine was 4.5 mg/dL. Ultrasound showed a left contracted echogenic kidney and a normal-sized right kidney with well-maintained cortico-medullary differentiation. He underwent a right renal biopsy, which showed normal glomeruli with mild tubulointerstitial fibrosis. He presented to our hospital for a second opinion with serum creatinine 2.67 mg/dL, hemoglobi 12.3 g/dL, potassium 3.34 meq/L, and bicarbonate 33.5 meq/L. Doppler showed a right-side parvus tardus pattern with high peak systolic velocity at the origin of the right renal artery, suggestive of severe right RAS. Marked wall calcification was seen in the infra-renal aorta. The bilateral external iliac artery appeared normal. Multi-slice spiral computed tomography (MSCT) renal angiography showed a left contracted kidney with severe stenosis of a 7-mm segment at the origin of the left renal artery with poor vascularity. Severe stenosis of a 5-mm segment of the proximal right renal artery just distal to the origin was seen [Figure 1].
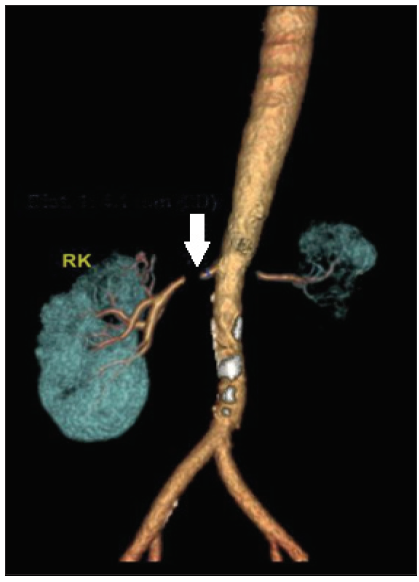
- MSCT renal angiography showing near complete occlusion of the right renal artery of 5 mm (white arow). The left kidney is contracted with severe stenosis of the left main renal artery at its origin measuring 7 mm. RK: Right Kidney, MSCT: Multi-slice spiral computed tomography.
Aortogram done via brachial approach showed complete total occlusion of the right renal artery [Figure 2]. A 0.014 in x 180 cm stiff guide wire (Brand: Shinobi) was manipulated across the stenosis with the support of a microcatheter (Progreat). This was exchanged for a balloon angioplasty catheter. Balloon dilatations were done with a 2 mm × 10 mm balloon. Follow-up arteriography revealed mild residual stenosis, which was stented with 5 mm × 18 mm and 5 mm × 12 mm RENOFIT stent. Follow-up dye study revealed complete perfusion of the right kidney. At 2 months post procedure, his BP was 120/80 mmHg on a single antihypertensive, and renal function improved to 0.98 mg/dL.
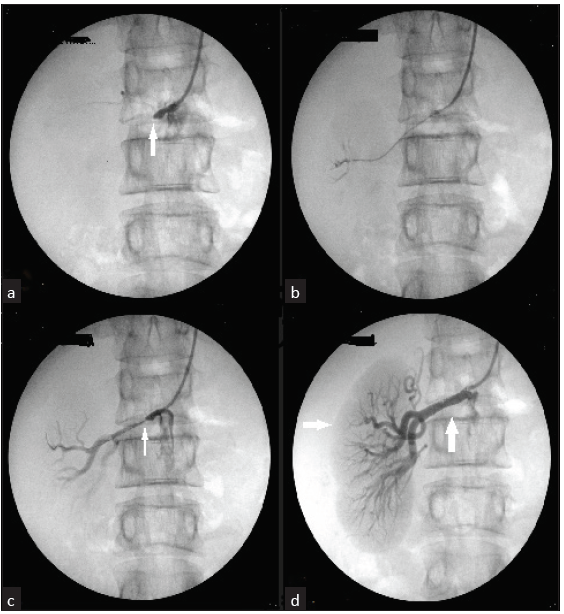
- (a) Aortogram via the right brachial artery showing complete occlusion of the right renal artery (white arrow). (b) A 0.014i x180 cm stiff guide wire (Shinobi: Brand) passed through the stenosis with the support of a microcatheter (Progreat Catheter). (c) Angiography after balloon dilatation with 2 mm x 10 mm showing mild residual stenosis (white arrow). (d) Showing a completely perfused kidney (horizontal white arrow) after Deploying two RENOFIT Stents of 5 mm x 18 mm and 5 mm x 12 mm in the right renal artery (vertical white arrow).
Discussion
Furthermore, 30%–50% of cancer treatment protocols include radiation either alone or with chemotherapy. Severe hypertension associated with radiation of the kidneys is frequently seen either in the acute phase or remotely.2
Arteries in the field of irradiation are at risk such as coronary artery disease after radiation to the chest for lymphoma or breast cancer,3 and carotid artery disease after irradiation to the head and neck. Radiation-induced enteritis, mesenteric ischemia, and RAS are reported following radiation to the abdomen and pelvis.
The median time to develop RAS is anywhere between 1 and 9 years, with an incidence of 0.5 to 10 cases per 1000 cases as a cause of hypertension.3 The exact pathophysiology is unknown, but radiation causes cell cycle arrest and ROS-induced DNA damage to rapidly proliferating endothelium,4 induces the release of Thromboxane, Von Willebrand factor, and platelet adhesion predisposing to arterial thrombosis.5 Increased pro-inflammatory cytokines such as interleukin-1, IL-6, TNF-a, and TGF-b induce the transformation of fibroblast to matrix-producing myofibroblast, leading to connective tissue proliferation, periarterial fibrosis, and accelerated atheroma formation.6 The aorta, proximal segment, and renal artery ostium are involved due to increased shear stress or irradiation of lymphnodes in proximity to the renal hilum. Treatment is either percutaneous intervention, bypass surgery, or nephrectomy. Our patient had no other risk factors for atherosclerosis. Endovascular procedure was chosen as it has lower perioperative morbidity and shorter hospital stay. Long-term patency is better with surgical repair with the added risk of surgical challenge due to distorted anatomy in prior field of irradiation.
We suggest that patients who develop hypertension after abdominal radiation should be evaluated for RAS, and PTCA with stenting is effective.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- The global burden of cancer: Priorities for prevention. Carcinogenesis. 2010;31:100-10.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hypertension in radiation nephritis. Report of a patient with unilateral disease, elevated renin activity levels, and reversal after unilateral nephrectomy. Arch Intern Med. 1977;137:848-51.
- [CrossRef] [PubMed] [Google Scholar]
- [Renal artery stenosis after abdominal radiotherapy] Ann Cardiol Angeiol (Paris). 2009;58:183-6.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of ionizing radiation induced damage of endothelial progenitor cells in vascular regeneration. Arterioscler Thromb Vasc Biol. 2012;32:343-52.
- [CrossRef] [PubMed] [Google Scholar]
- Ionizing radiation enhances platelet adhesion to the extracellular matrix of human endothelial cells by an increase in the release of von Willebrand factor. Radiat Res. 1994;137:202-7.
- [PubMed] [Google Scholar]
- Sensitisation of blood vessels to hypertensive damage by x-irradiation. Lancet. 1961;1:580-3.
- [CrossRef] [PubMed] [Google Scholar]








