Translate this page into:
Rapidly progressive glomerulonephritis in a patient with renal amyloidosis: Case report and review of the literature
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Renal amyloidosis is characterized by progressive deposition of extracellular material, most commonly in the glomeruli. Most often, patients present with nephrotic range proteinuria and the disease progresses gradually to renal failure. Rapid worsening of renal functions is rare. We report a case of crescentic glomerulonephritis superimposed on amyloidosis, clinically presenting as rapidly progressive renal failure, and present a review of the literature.
Keywords
Amyloidosis
crescents
rapidly progressive renal failure
Introduction
Amyloidosis of the kidneys is caused by deposition of amyloid protein. Glomeruli are involved in 75-90% of cases, clinical correlate of which is nephrotic-range proteinuria and slow progression to chronic kidney disease. Rapid progression of renal dysfunction over days is rarely seen. It is seldom appreciated that extracapillary glomerulonephritis may be superimposed on amyloidosis in such cases. We report one such rare case.
Case Report
A 50-year-old man presented with the complaint of swelling of feet and facial puffiness of 1 month duration accompanied by oliguria. There was no hematuria or turbid urine. There was no history of exertional breathlessness or orthopnea. His appetite was fairly well preserved and his bowel movements were regular. He was not a diabetic or hypertensive and had no significant illness in the past.
On examination, he had pallor, pitting pedal edema, and facial puffiness. His blood pressure was 120/80 mmHg. Respiratory system examination revealed bilateral basal crepitations. Abdominal examination showed the presence of ascites without any evidence of organomegaly. Laboratory examination showed hemoglobin of 10 mg/ dL, total leukocyte count of 14.4 × 109/L with 76% of polymorphs in differential count. Biochemical parameters were as follows: random blood sugar 127 mg/dL, serum creatinine 1.4 mg/dL, blood urea 34 mg/dL, and serum albumin 0.9 g/dL. Serum electrolytes and liver function tests were normal. Erythrocyte sedimentation rate was 140 mm/h. Urine analysis showed 3+ protein, 2–3 pus cells/hpf, and inactive sediments. Urine protein creatinine ratio was 4.6 g/g. Chest X-ray showed bilateral pleural effusion. Abdominal sonogram showed moderate ascites with bilateral pleural effusion and bilateral normal-sized kidneys.
Serum protein electrophoresis showed normal pattern lacking “M-spike.” Renal biopsy was carried out to ascertain the nature of glomerular pathology. Light microscopy showed 24 glomeruli, all of which showed pale, eosinophilic, acellular, weakly periodic acid-Schiff (PAS)-positive material in the mesangium and basement membrane, focally forming nodules [Figure 1]. Congophilia and apple-green birefringence were documented. There was no interstitial fibrosis or tubular atrophy. Minimal amount of amyloid material was seen in the wall of interlobular arteries. Immunofluorescence showed ten viable glomeruli that were negative for IgG, IgA, IgM, C3c, C1q, and fibrin. Immunohistochemical studies were positive for amyloid A protein in the deposits [Figure 2]. There was no light chain restriction. Bone marrow examination and skeletal profile ruled out plasma cell dyscrasia. Serum rheumatoid arthritis (RA) factor was negative. A diagnosis of renal amyloid A (AA) amyloidosis causing secondary nephrotic syndrome was reached and the patient was put on enalapril, atorvastatin, and diuretics. He was also initiated on colchicine 1.2 mg/day.

- Three glomeruli displaying global deposition of pale eosinophilic acellular weakly PAS-positive material deposited in the mesangial region and along capillaries, obliteration of capillary lumen and patent bowmans’ space. Scanty amyloid material is seen in the vessel wall (20×, PAS)
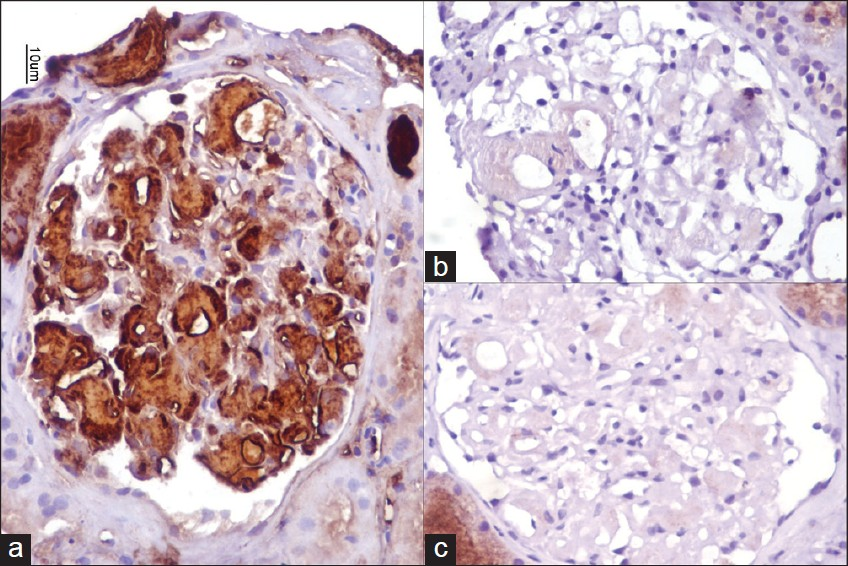
- Amyloid deposits are strongly positive for amyloid A protein (a), and lack of light chain restriction with kappa (b) and lambda (c) light chains (40×, Immunoperoxidase)
He returned for follow-up after 1 month with complaints of anorexia, nausea, and oliguria. He had discontinued treatment 3 weeks before. On evaluation, he had anasarca and had blood pressure of 130/80 mmHg. Clinically, the patient had persisting bilateral pleural effusion and ascites. Laboratory tests were as follows: hemoglobin 6.5 g/dL, blood urea 157 mg/dL, serum albumin 1.8 g/ dL, and serum creatinine 6.5 mg/dL. Urine analysis showed 3+ protein and 10–12 RBCs/hpf. RBC casts and granular casts were seen. Doppler study ruled out renal vein thrombosis. Antineutrophil cytoplasmic antibody (by immunoblot assay) was negative and serum C3 was 93.8 mg/dL (normal, 90–180 mg/dL). Considering the rapid deterioration of renal function and sudden drop in hemoglobin, renal biopsy was repeated. Fourteen viable glomeruli were seen on light microscopy, of which 30% of them showed active cellular crescents occupying circumferentially in addition to amyloid deposits [Figure 3]. There was no fibrinoid necrosis of the tufts. Vessel wall had minimal amyloid deposits and no area of necrotizing vasculitis was seen. Immunofluorescence showed eight viable glomeruli that were negative for IgG, IgA, IgM, C3c, C1q, and fibrin. There was no light chain restriction with kappa and lambda light chains.
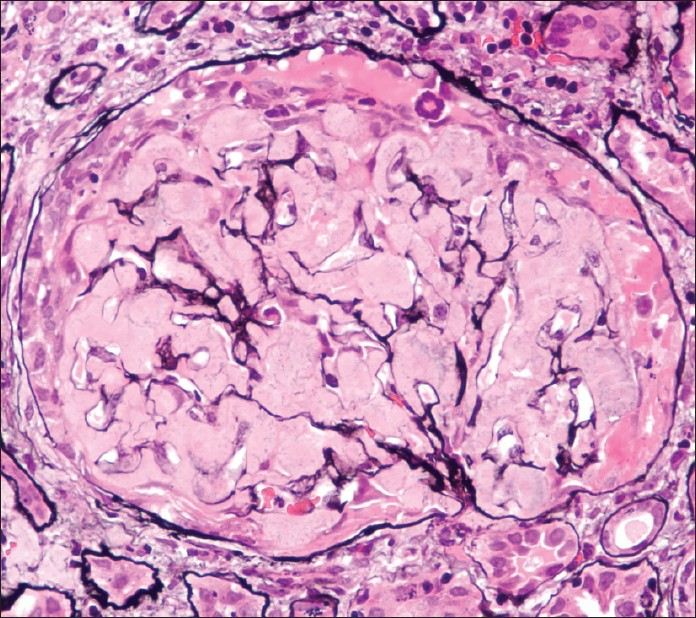
- Repeat biopsy: Glomerulus shows global deposition of amyloid material that is silver-negative and an active cellular crescent seen circumferentially along with fibrinous material in the crescentic area (40×, PASM stain)
The patient was treated with large doses of diuretics. Injectable methylprednisolone (1g) was given on three consecutive days. Inj. cyclophosphamide 500 mg was given. The patient gradually had increasing urine output and was symptomatically better at discharge, with serum creatinine improving to 3.5 mg/dL. Unfortunately, he was lost to follow-up.
Discussion
Amyloidosis of the kidney is characterized by deposition of amyloid protein in the renal parenchyma. Clinical manifestations vary depending on the predominant site of amyloid deposition. Glomeruli are involved in 75-90% of cases. Patients present with heavy proteinuria and a progressive course, leading to slow decline in renal functions and end-stage renal disease. Treatment involves reduction of amyloid precursor protein. This may be in the form of ablation of plasma cells in myeloma-associated amyloidosis, or suppression of acute phase response and treatment of underlying chronic inflammatory/infective condition in AA amyloidosis.[1] Colchicine has been used in amyloidosis associated with familial Mediterranean fever with good results. Eprodisate is a new drug being tried in secondary amyloidosis.[2] With all these different therapies, the course of the decline in renal functions can be further slowed down or even arrested.
Rapid decline of renal functions is unusual and is attributed to the severe nephrotic state causing prerenal type of renal insufficiency. Progressive deposition of amyloid itself has been found to be the cause in a few cases.[3] Rapidly progressive glomerulonephritis (RPGN) in the background of amyloidosis is distinctly rare and has been described in anecdotal reports.[4] Panner reported the first two cases of possible renal amyloidosis that presented as RPGN, one of whom had RA.[5] There are some other reports of rapidly progressive renal failure in patients with longstanding RA that demonstrated amyloidosis on renal biopsy, associated with crescentic glomerulonephritis.[67] Crescents have been described in AL amyloidosis as well. In a recent report, Crosthwaite et al. have reported one case of crescentic glomerulonephritis in a case of AL amyloidosis, which is probably the first report of an association of AL amyloidosis with crescents.[8]
The pathophysiology of crescents in amyloidosis is speculative. Nagata et al. have proposed that damage to the glomerular basement membrane (GBM) ultrastructure by the amyloid fibrils led to crescent formation.[9] In their series of 105 cases of renal amyloidosis studied on autopsy or biopsy, glomerular crescents were seen in 14 cases (13%). Immunohistochemical studies among these cases documented amyloid protein of AA type in 12 cases (85%). They noted that RA was the most frequent primary disease associated with amyloidosis. They recorded that crescent formation was more closely associated with the site of amyloid deposition and not with the level of proteinuria, with mesangial deposition being more commonly associated with crescents. Electron microscopic studies showed areas of the GBM disruption in areas of amyloid deposition supporting their hypothesis. Leakage of fibrin-fibrinogen caused by gaps in the GBM incites inflammatory reaction and plays a crucial role in extracapillary proliferation.[10] It is also likely, however, that crescents may be of a different pathophysiology and the association with renal amyloid may be incidental.[11] In another case report, a patient with chronic inflammatory bowel disease treated with infliximab developed rapidly progressive renal failure 3 months later. On renal biopsy, crescents and vasculitis, presumably due to infliximab, were seen in addition to AA amyloidosis.[12] In our patient, the cause for crescent formation was not clear. We considered the crescentic transformation was either due to vasculitis in view of the sudden drop in hemoglobin and renal insufficiency or drug (colchicine) induced. However, the ANCA profile was negative and the literature is silent with regard to colchicine associated with crescent formation. On the contrary, there are reports that suggest that colchicine is an immunomodulatory drug and downregulates inflammatory response.[1314] The patient was treated with fresh frozen plasma in addition to ACE inhibitors in an effort to alleviate the nephritic edema and third-space collections. In addition, as the renal function declines and urine output drops, the quantity of urinary protein loss comes down, which could possibly explain the improvement in serum albumin levels.
It is possible that association of crescents in a proven case of amyloidosis may be more common than previously considered.[9] The association may be missed as such patients may not be subjected to a repeat biopsy even when they present with rapid deterioration in renal function.[6]
Treatment of RPGN associated with amyloidosis is not clear. The benefits of aggressive immunosuppression with a combination of steroids and cytotoxic drugs in patients who are already protein depleted and malnourished are not well documented and may cause more harm than good. It appears that that these patients do worse than patients with RPGN without underlying amyloidosis. However, there are isolated case reports that indicate that aggressive treatment may salvage the kidney function, at least partially. There is one case report of RPGN associated with amyloidosis in a 53-year-old lady with RA who was successfully treated with intensive plasma exchange and immunosuppression. In this patient, the renal function improved and hemodialysis could be discontinued. The renal function remained stable at 2 years, although heavy proteinuria persisted.[15] Moroni et al., in their report of three cases of crescentic glomerulonephritis superimposed on amyloidosis, have documented partial recovery of renal functions in two patients after treatment with intravenous pulse methylprednisolone, immunosuppressive agents, and oral corticosteroids.[16]
To the best of our knowledge, this is the first ever such association described from India. Our patient had AA amyloidosis as proven by immunohistochemical studies, but no underlying disease could be identified. He was treated with pulse methylprednisolone along with IV cyclophosphamide, but had nephrotic-range proteinuria and moderate renal dysfunction till last follow-up.
In summary, crescentic glomerulonephritis associated with renal amyloidosis is a rare occurrence and it is important that clinicians suspect this possibility when confronted with a case of renal amyloidosis and rapid worsening of renal functions. Early recognition and prompt treatment may be beneficial in the salvage of renal functions.
We sincerely thank Mrs. Tulasi Kumari, Mrs. Hema Nagaraj, Mr. Nagaraj, and other members of the histopathology section.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Amyloidosis. In: Davison AM, Cameron JS, Grünfeld JP, Ponticelli C, Ritz E, Winearls C, eds. Oxford Textbook of Clinical Nephrology (3rd ed). Oxford: Oxford Publications; 2008. p. :679-701.
- [Google Scholar]
- Eprodisate for the treatment of renal disease in AA amyloidosis. N Engl J Med. 2007;356:2349-60.
- [Google Scholar]
- Secondary amyloidosis and rapidly progressing renal insufficiency.A clinico-pathological study. Med Clin (Barc). 1991;97:557.
- [Google Scholar]
- Crescentic glomerulonephritis. In: Davison AM, Cameron JS, Grünfeld JP, Ponticelli C, Ritz E, Winearls C, eds. Oxford Textbook of Clinical Nephrology. Oxford: Oxford Publication; 2008. p. :559-78.
- [Google Scholar]
- Rapidly progressive glomerulonephritis and possible amyloidosis. Arch Path Lab Med. 1980;104:603-9.
- [Google Scholar]
- Renal failure in a patient with two renal diseases: renal amyloidosis and rapidly progressive glomerulonephritis. Nephrol Dial Transplant. 1997;12:341-3.
- [Google Scholar]
- Renal amyloidosis associated with crescentic glomerulonephritis. Am J Nephrol. 1984;4:52-5.
- [Google Scholar]
- Rapidly progressive glomerulonephritis complicating primary AL amyloidosis and multiple myeloma. Nephrol Dial Transplant. 2010;25:2786-9.
- [Google Scholar]
- Glomerular crescents in renal amyloidosis: an epiphenomenon or distinct pathology? Pathol Int. 2001;51:179-86.
- [Google Scholar]
- The patient with two renal diseases: crescentic glomerulonephritis and renal AA amyloid. Nephrol Dial Transplant. 1999;14:1315-6.
- [Google Scholar]
- Renal amyloidosis associated with extracapillary glomerulonephritis and vasculitis in a patient with inflammatory bowel disease treated with infliximab. Clin Nephrol. 2008;70:240-4.
- [Google Scholar]
- Induction of nephrotoxic serum nephritis in inbred mice and suppressive effect of colchicine on the development of this nephritis. Pharmacol Res. 2002;45:319-24.
- [Google Scholar]
- Moderate protection of renal function and reduction of fibrosis by colchicine in a model of anti-GBM disease in the rabbit. J Am Soc Nephrol. 1990;1:257-65.
- [Google Scholar]
- Rapidly progressive glomerulonephritis associated with amyloidosis: efficacy of plasma exchange. J Clin Apher. 1987;3:226-9.
- [Google Scholar]
- Extracapillary glomerulonephritis and renal amyloidosis. Am J Kidney Dis. 1996;28:695-99.
- [Google Scholar]







