Translate this page into:
Rare Occurrence of PLA2R-Positive Membranous Nephropathy in a Patient with Sjogren's Syndrome and CIDP
Address for correspondence: Dr. Dinesh Khullar, Max Superspeciality Hospital, Saket, New Delhi, India. E-mail: nimish392005@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Membranous nephropathy is known to be associated with number of autoimmune diseases. Occurrence of PLA2R positive membranous nephropathy with sjogren's syndrome and chronic inflammatory demyelinating neuropathy (CIDP) is quite rare. Role of PLA2R antigen in autoimmune diseases like sjogren's syndrome and CIDP is largely unknown. Choice and initiation of immunosuppression if required may also be governed by the presence of other autoimmune diseases along with PLA2R positive membranous nephropathy
Keywords
Chronic inflammatory demyelinating neuropathy
membranous nephropathy
podocyte phospholipase A2 receptor
Sjogren's syndrome
Introduction
Membranous nephropathy is increasingly being reported with a number of autoimmune diseases. Primary membranous nephropathy is associated with IgG4 antibodies against podocyte phospholipase A2 receptor (PLA2R) antigen in 70% of cases, serum anti-THS7DA antibodies in 3%–5% of cases, and biopsy staining of PLA2R antibodies without presence of serum antibodies in 15% of cases.[1] A number of case reports have described the concurrence of chronic inflammatory demyelinating polyneuropathy (CIDP) and membranous nephropathy.[2] Autoantibodies against the paranodal proteins in nerves, such as neurofascin 155 (NF155), contactin-1 (CNTN1), and contactin-associated protein 1 (CASPR1), were seen to be present among subsets of patients with CIDP.[2]
In Sjogren's syndrome kidney involvement is frequent, predominantly involving the tubulointerstitial compartment rather than glomeruli.[3] Membranous nephropathy is the most common glomerular disease in patients with Sjogren's syndrome.[3] Although there are case reports of patients with Sjogren's syndrome having CIDP, occurrence of PLA2R-positive membranous nephropathy, Sjogren's syndrome, and CIDP all in one patient is quite rare. In a single case report of patient of biopsy-proven membranous nephropathy, Sjogren's syndrome and CIDP were positive for anti-CNTN antibodies and negative for PLA2R.[2]
Case History
A 56-year-old businessman who was hypertensive from the past 17 years and diabetic for the past 4 years presented with insidious onset swelling of both the feet along with frothy urine. Diabetes was well-controlled on metformin and he was compliant with his antihypertensive medication cilnidipine 10 mg once daily. His blood pressure was 134/82 mmHg and pulse 86/min regular. On investigation, urine routine microscopy revealed pH of 5.3 and specific gravity of 1.012 along with 3+ proteinuria with no red blood cells and white blood cells. He had nephrotic range proteinuria of 4 g/day in 24-h urinary protein estimation. His hemoglobin was 12.78 g/dL, total leucocyte count 5900/mm3, and serum creatinine 0.4 mg/dL with serum urea of 15 mg/dL. He was advised for renal biopsy for which he declined and was started on ACE inhibitors with close monitoring.
After 2 months, he developed gradual onset tingling sensation along with weakness in both lower limbs which involved both the feet first and gradually involving the whole of lower limb till groin. Weakness was insidious in onset and progressive in nature and was first noticed by patient while difficulty in wearing slippers. Weakness and tingling sensation gradually involved both the hands as well progressing involving both the upper limbs till shoulder joints. Weakness progressed to the stage where the patient was able to stand or walk only by support. There was no history of decreased sensation of clothing and no history of inability to appreciate hot and cold. During this time, his swelling also progressed to involve the entire body and proteinuria increased to 16 g/day.
On examination, he had hypotonia in both upper and lower limbs. In both the hip joints, power was reduced 2/5 in flexion, extension, adduction, and 4/5 in abduction. At knee joint in both flexion and extension, power was 4/5. Power in both the upper limbs was 4/5 in flexion at elbow and wrist joint and 5/5 in extension. Sensory examination was normal except bilateral impaired position sense at toe. Plantar reflex was bilateral flexor, and delayed tendon reflexes were absent in both the upper limbs.
He was advised for renal biopsy for which he agreed eventually. In light microscopy of kidney biopsy, 13 of 14 glomeruli revealed diffuse thickening of glomerular basement membrane suggestive of membranous nephropathy [Figure 1]. Immunohistochemistry showed diffuse granular positivity for PLA2R antigen along glomerular capillary walls [Figure 2]. In direct immunofluorescence, granular deposits of IgG were 3+, IgM and IgA were negative, C3 was 1+, C1q was negative, and both kappa and lambda light chain 3+ granular deposits were present along capillary wall. Biopsy was suggestive of membranous nephropathy with interstitial fibrosis and tubular atrophy (IFTA) of around 10%–15%. Serum PLA2R Ab was negative. Viral markers were sent which were negative. Nerve conduction velocity revealed predominantly demyelinating sensory motor polyneuropathy more in lower limbs than in upper limbs consistent with CIDP. Compound muscle action potential in bilateral tibial nerves was not recordable, depressed in bilateral median, right, ulnar, and peroneal nerves. Sensory nerve action potential of bilateral median, ulnar, and sural nerves was not recordable. F-waves were prolonged in bilateral median ulnar and peroneal nerves. CIDP was diagnosed as per clinical criteria and American Academy of Neurology electrodiagnostic criteria.[4]

- Renal Biopsy shows uniform thickening of glomerular capillary walls which is suggestive of Membranous nephropathy
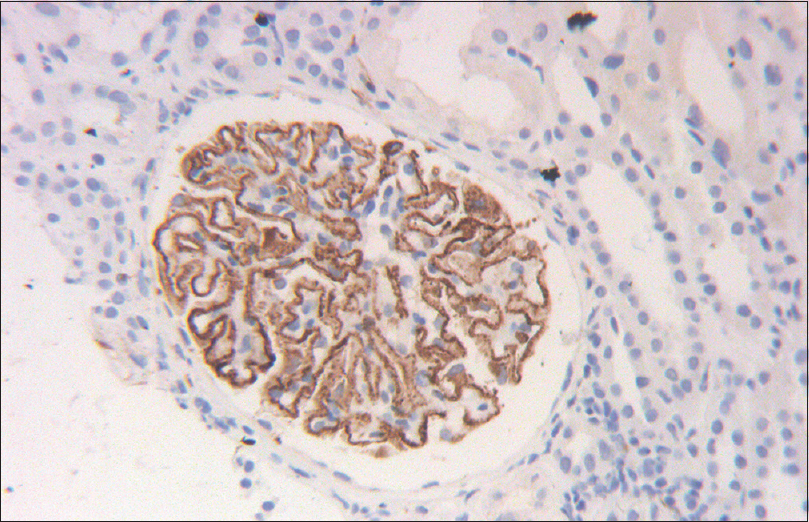
- Renal biopsy shows uniform staining of glomerular capillary walls with PLA2R antigen
The patient also complained of dryness in eyes and dry mouth and subsequently underwent Schirmer's test which was positive. ANA by immunofluorescence was negative, and C3 and C4 levels were normal. Anti-rho/anti-la was negative. Salivary gland biopsy was done which revealed chronic sialadenitis [Figure 3] with immunohistochemistry revealing CD45 +ve rich cells [Figure 4]. Hence, the patient was diagnosed with Sjogren's syndrome as per 2016 ACR/EULAR criteria.[5]
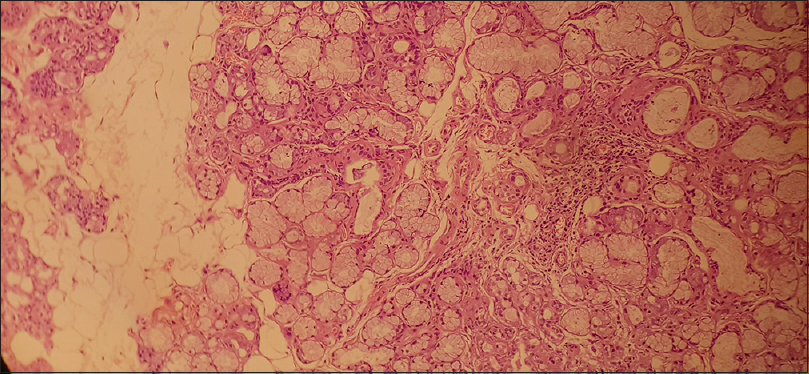
- Salivary gland tissue with peri-acinar lymphocytic cell infiltration suggestive of Sjogren's syndrome
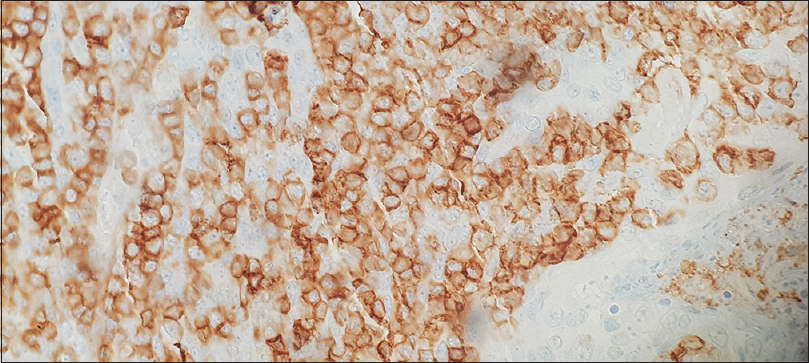
- Salivary gland shows CD 45 positive lymphocytic cell infiltrates suggestive of Sjogren's syndrome
IgG4 substaining was also done which shows positive IgG4-positive intramembranous deposits [Figure 5]. He underwent 18F-FDG whole-body positron emission tomography–computed tomography to rule out malignancy which was negative. He was started on oral prednisolone (1 mg/kg) along with ACE inhibitors/statin and received high-dose intravenous immunoglobulin (IVIG) (2 g/kg) but weakness did not improve. He received injection rituximab 375 mg/m2 as four doses once weekly along with cotrimoxazole antibiotic prophylaxis.
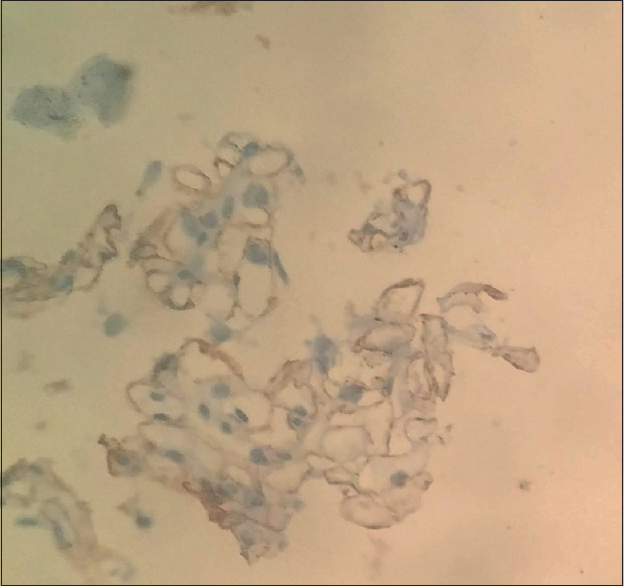
- Positive IgG4 staining along glomerular capillary wall suggestive of Primary membranous nephropathy
After 4 months of follow-up, weakness and proteinuria of 9 g/day were still persisting. CD 19 counts monitored were 0% after 4 months. He was continued on low-dose steroids with 10 mg prednisolone once daily, but his weakness started to worsen gradually along with persistent proteinuria of around 9.8 g/day. He was started on oral cyclophosphamide (2.5 mg/kg) dose, and the dose was modified as per total leucocyte count every 14 days. He continued to receive prednisolone 10 mg daily.
He was again started cotrimoxazole prophylaxis and he was monitored for cytomegalovirus infection via serum CMV PCR. He received cyclophosphamide for 4 months and gradually weakness improved to power 4+/5 in distal limbs and proteinuria also gradually improved to 1.62 g/day. Currently, the patient is on 10 mg prednisolone and is being closely monitored for relapse.
Discussion
Occurrence of PLA2R-positive membranous nephropathy, Sjogren's syndrome, and CIDP in one patient is quite rare. Biopsy-positive PLA2R membranous nephropathy with negative serum PLA2R is likely due to “sink effect” and can be present in 15% of primary membranous nephropathy.[1]
There are only few case reports showing PLA2R +ve membranous nephropathy with Sjogren's syndrome.[6] Membranous nephropathy in certain diseases such as malignancy, hepatitis C–positive patients, and sarcoidosis have higher than expected PLA2R positivity.[7] Abnormal T- and B-cell activation in patients with sarcoidosis and hepatitis C is a proposed mechanism,[7] and role of PLA2R antibodies in other autoimmune mechanism still needs to be elucidated.
The choice of immunosuppression in primary membranous nephropathy may also be governed by the presence of other concomitant autoimmune disease. Various immunosuppression regimes have been described in cases of CIDP with primary membranous nephropathy.[8] In this case, rituximab was used followed by cyclophosphamide in case of worsening neurological deficit as cyclophosphamide shows good response in refractory CIDP and in membranous nephropathy.[14]
Diseases mediated by antibodies of the IgG4 isotype like PLA2R-positive membranous nephropathy showed good response to anti-B-cell therapy; in a recent randomized controlled trial, rituximab showed significantly more partial remission rates than placebo in a mean period of 17 months (65% vs. 34%, P < 0.01).[1] Similarly, other diseases with anti-IgG4 isotype such as myasthenia gravis with anti-muscle-specific tyrosine kinase (MusK) antibodies and pemphigus vulgaris respond well to B-cell-depleting therapies.[9]
CIDP with IgG4 anti-CNTN1 antibodies account for less than 10% of all patients with CIDP.[9] There are case reports showing good response and dramatic improvement with rituximab in patients with CIDP with IgG4 anti-CNTN1 antibodies who were resistant to IVIG and corticosteroids.[9]
In this case, IVIG was given first and then in case of inadequate response four doses of rituximab were given. In view of persistent neurological worsening and persistent nephrotic range proteinuria, cyclophosphamide was given after which the patient improved. Whether it was delayed effect of rituximab, it cannot be commented upon.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Chronic inflammatory demyelinating polyneuropathy with concurrent membranous nephropathy: An anti-paranode and podocyte protein antibody study and literature survey. Front Neurol. 2018;9:997.
- [Google Scholar]
- Sjögren syndrome-related membranous glomerulonephritis progressing to membranoproliferative glomerulonephritis. Case Rep Nephrol Dial. 2016;6:133-42.
- [Google Scholar]
- An update on the management of chronic inflammatory demyelinating polyneuropathy. Ther Adv Neurol Disord. 2012;5:359-73.
- [Google Scholar]
- The 2016 classification criteria for primary Sjogren's syndrome: What's new? BMC Med. 2017;15:69.
- [Google Scholar]
- Anti-PLA2R antibody in a secondary cause of membranous nephropathy. Nephrology. 2018;23:94-5.
- [Google Scholar]
- Anti-PLA2R-associated membranous nephropathy: A review with emphasis on diagnostic testing methods. Clin Nephrol. 2015;84:1-9.
- [Google Scholar]
- Glomerulonephritis associated with chronic inflammatory demyelinating polyneuropathy. Ren Fail. 2006;28:255-9.
- [Google Scholar]
- Rituximab in treatment-resistant CIDP with antibodies against paranodal proteins. Neurol Neuroimmunol Neuroinflammation. 2015;2:e149.
- [Google Scholar]







