Translate this page into:
Recent advances in anti-neutrophil cytoplasmic antibody-associated vasculitis
Address for correspondence: Dr. D. Ranganathan, Department of Kidney Health Services, Royal Brisbane and Women's Hospital, Herston, Queensland - 4029, Australia. E-mail: Dwarakanathan.ranganathan@health.qld.gov.au
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Anti-neutrophil cytoplasmic antibody-associated vasculitis is an uncommon inflammatory disease of small to medium-sized vessels that frequently presents with rapidly progressive glomerulonephritis and renal failure though it can affect any organ system. If untreated, the vast majority of patients will die within a year. Current treatments improve prognosis but affected patients remain at a substantially higher risk of death and adverse outcomes. We review the classification of the disease, our understanding of the pathogenesis and epidemiology, and propose future directions for research. We also evaluate the evidence supporting established treatment regimens and the progress of clinical trials for newer treatments to inform the design of future studies.
Keywords
Anti-neutrophil cytoplasmic antibodies
associated vasculitis
renal
Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a disease process characterized by necrotizing inflammation of small vessels, the relative paucity of immune deposits and an association with detectable circulating ANCAs. Three subtypes have been described; granulomatosis with polyangiitis (GPA, formerly Wegener's granulomatosis), microscopic polyangiitis (MPA), and eosinophilic GPA (EGPA, formerly Churg-Strauss syndrome).
Collectively, these disorders account for a considerable burden of death and disability worldwide and are of great clinical importance owing to the vast improvement in prognosis with treatment. As a result, AAV has been studied extensively in recent years. This review aims to clarify the state of the science, summarize recent developments in classification, epidemiology, pathogenesis and treatment, and to identify knowledge gaps and future research priorities in AAV.
Classification and diagnosis
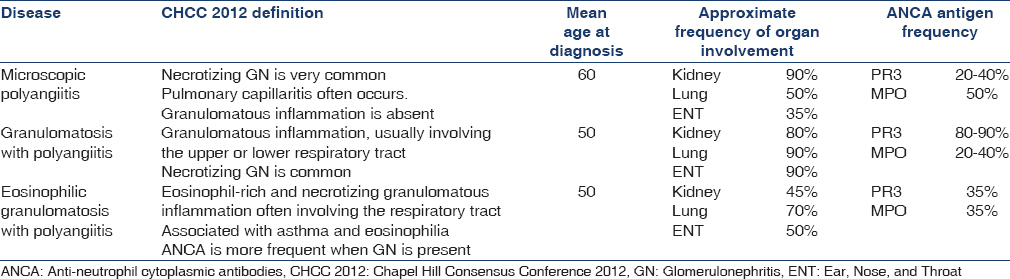
In the absence of a known cause, the vasculitides are first classified according to the size of the vessels that are predominantly involved. AAV predominantly affects small vessels, defined as small intraparenchymal arteries, arterioles, capillaries, and venules.[1] Distinguishing between the different forms of AAV, particularly GPA and MPA, has been a longstanding problem for clinicians and researchers alike.[2] This is partly because MPA was not recognized as a unique entity when the American College of Rheumatology (ACR) diagnostic criteria were developed in 1990. Similarly, while the Chapel Hill Consensus Conference 2012 definitions highlight that granulomatous inflammation in the respiratory tract is a key distinguishing feature of GPA, these definitions are not designed as diagnostic criteria [Table 1].
There has been conjecture as to whether GPA and MPA subtypes could be combined under the umbrella of AAV. It has been argued that distinguishing between the diagnoses of GPA instead of MPA confers only minimal additional prognostic information,[34] and distinguishing between them in the clinical setting is often difficult or unnecessary, as both conditions have been combined in clinical therapeutic trials.[5] Five subtypes of AAV – renal AAV with proteinase 3-ANCA (PR3-ANCA), renal AAV without PR3-ANCA, nonrenal AAV, cardiovascular AAV, and gastrointestinal AAV, based on the phenotypic spectrum was proposed by French and European Vasculitis group but require further validation.[6]
There are, however, many epidemiological and genetic studies suggesting that GPA and MPA are two distinct pathological processes, which could warrant different treatment modalities in the future.[6] For example, studies suggest that infection is a trigger for GPA, but not MPA and that geographical variations in incidence may relate to environmental or underlying genetic differences.[6]
While differentiating EGPA from GPA or MPA is usually more straightforward, diagnosis of the disease itself can be more challenging and is often delayed due to its typically phasic nature, with onset of asthma preceding development of vasculitis by approximately 8–10 years and naso-sinus disease often occurring before development of eosinophilia.[7] However, the 1990 ACR are only relevant for the classification of EGPA in a patient with existing vasculitis and established criteria to facilitate early diagnosis of EGPA are lacking.
The Diagnostic and Classification Criteria for Vasculitis (NCT01066208) study aim to address this need for reliable diagnostic criteria,[8] in keeping with proposed guidelines by the ACR and the European League Against Rheumatism. It is a multinational observational cohort study that has completed recruitment of over 2000 cases and 490 controls to delineate best and validate a set of diagnostic criteria for six primary vasculitides; GPA, MPA, EGPA, polyarteritis nodosa, giant cell arteritis and Takayasu arteritis. Detailed clinical data, measurement of ANCA, biopsy and imaging data will be included in a multivariate model that delineates key distinguishing features between these conditions and other autoimmune conditions that may present with similar features. Predefined cases to establish a reference standard for each condition and data driven classification algorithms will be used in this study, and have demonstrated superiority to physician-opinion as the gold standard for diagnosis.[8]
Positivity for ANCA, while useful, is unfortunately not a perfect marker for AAV. Between 10% and 20% of untreated patients with GPA or MPA do not have positive ANCA, and up to 60% of patients with EGPA are ANCA negative.[910] Identifying new biomarkers would simplify the task of delineating between primary vasculitides. Three promising biomarkers for distinguishing AAV and non-AAV patients, based on existing definitions, have been identified; matrix-metalloproteinase-3, tissue-inhibitor of metalloproteinase-1, and CXCL13.[11]
Epidemiology
Determining the precise incidence of AAV is challenging, given the variations in methods of defining a case, and the low incidence of the disease. A prospective comparison of the incidence of AAV in the Miyazaki prefecture of Japan, and the Norfolk and Norwich Hospital Catchment in the UK, estimated the annual combined incidence of GPA and MPA to be similar in both regions; approximately 20 cases per million.[12] The methodologies used to arrive at this estimate, however, assumed that migration in and out of the catchment was minimal and that all incident cases of the disease in this catchment were diagnosed at sampled hospitals. Methodologies to evaluate the validity of these assumptions were not reported.[12] Nonetheless, the estimate of 20 incident cases per million population per year is broadly consistent with other epidemiological studies, which also indicate that the peak age of onset is between 65 and 74 years and the disease is extremely rare in childhood.[1314] The incidence of biopsy-proven ANCA vasculitis may also be biased toward cases in which there is renal involvement. Such a dominant clinical trigger would lead to an underestimation of the prevalence of vasculitis.[1516]
It has also been observed that MPA, which is more commonly positive for myeloperoxidase (MPO)-ANCA and a perinuclear staining pattern (p-ANCA), was the predominant subtype in Japan, while GPA, which is more commonly positive for PR3-ANCA and a cytoplasmic staining pattern (c-ANCA), was the predominant subtype in the UK.[15]
Epidemiological data from India has been difficult to interpret, as vasculitic disorders are often misdiagnosed as tuberculosis and therefore probably underreported.[16] Primary vasculitic disorders account for <1% of the cases seen in rheumatology clinics in major tertiary care hospitals and the frequency of distribution of GPA and MPA among those patients were 13% and 3%, respectively, the majority of cases being Henoch-Schonlein purpura and Aortoarteritis.[17] GPA (54%) was diagnosed more frequently compared to MPA (18%) from Southern India.[18] There is little literature on vasculitis from the Indian subcontinent over the past 9 years; the field is likely to have shifted significantly with greater awareness and wider application of diagnostic tests.
Apparent differences in the epidemiology of GPA and MPA suggest that separate pathological processes may be responsible for each disease type, however further studies are required to elucidate these underlying processes. Multiple possible explanations have been explored and are discussed in the following section.
Pathogenesis
Since their discovery in 1985,[19] ANCAs have become increasingly implicated in the pathogenesis of AAV [Figure 1]. The discovery that administering MPO-ANCA to wild type mice consistently causes disease has strongly supported its pathogenic role, and the resulting animal model has facilitated research into underlying molecular mechanisms and potential interventions.[5] Evidence in humans has also supported the pathogenic role of ANCA. Patients treated with propylthiouracil, which is a MPO inhibitor used to treat hyperthyroidism, may go on to develop MPO-ANCAs and an AAV-like syndrome.[20] There is also evidence that the titer of ANCA may correlate with risk of disease relapse,[21] although the specific parameters that define this risk and its utility in a clinical setting are somewhat controversial.[22]

- Pathogenesis of anti-neutrophil cytoplasmic antibody-associated vasculitis. Infectious, genetic, and environmental risk factors (a) are implicated in exposing cytoplasmic proteins in the neutrophil (e.g., Proteinase 3 and Lysosome-associated membrane protein 2), and, following likely interaction with T- and B-lymphocytes, the subsequent development of autoantibodies. Autoantibodies may also be generated to an epitope that is complementary to the autoantigen, such as anti-sense Proteinase 3 (b), or via molecular mimicry, such as the bacterial adhesion molecule FimH. (c) A second hit, such as infection or loss of gene silencing, is often required to trigger disease. (d) Antineutrophil cytoplasmic antibodies induced neutrophil activation also activates the alternative complement pathway. (e) In addition to complement mediated microvascular injury, antineutrophil cytoplasmic antibodies also mediates endothelial damage by enhancing neutrophil-endothelial cell interactions and increasing neutrophil degranulation of cytotoxic agents and chemoattractants. PR3: Proteinase 3, LAMP-2: Lysosome-associated membrane protein 2, FimH: Subunit of enterobacterial fimbriae
Activation of the complement system via the alternative pathway is thought to be a primary mechanism by which ANCA mediates disease.[23] In animal models, neither C5 nor Factor B knockout mice manifest the disease. Furthermore, in wild-type mice administered anti-MPO antibodies, subsequent treatment with a C5 inhibitor effectively prevents disease.[5] This is in comparison to C4 knockout mice which develop vasculitis. Together these findings suggest that the alternative pathway, rather than the classical or lectin pathways, mediate ANCA related complement activation making it an ideal target for therapy.[5] Human case-control studies have also found that Factor Bb, C3a, C5a and final common pathway factors were increased in active cases relative to controls, further supporting the role of complement activation in AAV.[2324] Interestingly, C4d levels were also elevated in active cases relative to controls, but there was no difference between levels in active disease and remission.[2324] The significance of this finding requires further investigation. Given the evidence, it is logical to test the hypothesis of whether inhibiting the complement system would be a viable therapeutic modality. A Phase 2 clinical trial is currently underway, which is designed to evaluate the safety and efficacy of a C5aR inhibitor, CCX168, in patients with ANCA-associated renal vasculitis (NCT01363388). Early results of this trial have not shown inferiority of CCX168.[25]
However, while ANCA and activation of the complement pathway are probably key pathogenic factors, our understanding of the processes involved is not complete. For example, some people with detectable circulating ANCA may not express a disease phenotype.[26] Similarly, PR3-ANCAs demonstrate an ability to stimulate leukocyte activation in vitro, but unlike MPO-ANCA cannot produce a reliable animal model for the disease.[5] It has been hypothesized that the presence of ANCA alone, irrespective of specificity to MPO or PR3, is not sufficient to produce disease in humans. Instead a “second hit,” such as an inflammatory response stimulated by a concomitant respiratory tract infection, may be required to trigger the full disease process. This is consistent with the finding that stimulating neutrophils with cytokines such as tumor necrosis factor alpha (TNF-α) in vitro will stimulate them to express the autoantigens (MPO and PR3) onto the cell surface.[27] Autoantibodies may also play a role in the ANCA negative form of the disease. In a series of eleven ANCA negative patients with pauci-immune focal necrotizing glomerulonephritis, eight were found to possess antibodies to human lysosome-associated membrane protein-2 (hLAMP-2) that bind to the glomerular, but not the neutrophil hLAMP-2 antigen.[28]
The origin of ANCA is unclear. Genetic, epigenetic, environmental and infectious factors that are associated with AAV have been described, but a convincing model of disease pathogenesis remains elusive. Classic genetic linkage studies have demonstrated that a first degree relative of a patient with GPA is approximately 1.6 times more likely to develop the disease than a first degree relative of a similar individual who does not.[29] A wide spectrum of genes, predominantly involving immune function, has been found to confer small amounts of increased risk of the disease.[30] Chromatin modification of MPO and PR3, a measure of epigenetic modification, also appears to be depleted in ANCA patients compared to healthy controls.[31]
Chronic occupational exposure to environmental toxins, most notably silica, is associated with an increased risk of AAV.[20] This is based on case series reporting an apparent association between silica exposure in patients that subsequently developed ANCA vasculitis, mostly of the MPA subtype.[3233] It is hypothesized that this occurs due to the intense inflammatory response promoted by silica, which may promote neutrophil migration and simultaneous formation of antibodies against neutrophil components.
Infections are becoming increasingly recognized as a potential “second hit” required to break tolerance and cause autoimmunity. This may be a reason for the observed seasonal fluctuation in the incidence of GPA.[20] Furthermore, certain bacteria such as Staphylococcus aureus and Escherichia coli are hypothesized to promote the disease intrinsically. Studies show that 67% of patients presenting with GPA are nasal carriers of S. aureus and nasal colonization increases the risk of disease relapse between 1.6 and 31-fold.[34] Furthermore, randomized trials of trimethoprim-sulfamethoxazole (TMP-SXT) suggest this medication is an effective means of inducing remission,[35] although whether this effect relates to the antimicrobial effect or some other property of TMP-SXT is unclear. Molecular mimicry is proposed as the primary mechanism by which infectious agents may contribute to the disease process. For example, there is a strong homology between the bacterial adhesion protein FimH and a newly discovered ANCA called LAMP-2.[36] It has also been found that the antisense strand of the PR3 gene can produce a protein that permits autoantibodies to both itself (complementary PR3) and native PR3. Interestingly, complementary PR3 shows weak homology to S. aureus proteins.[30]
Treatment
Induction therapy
Induction therapy with a combination of high-dose steroids and cyclophosphamide has been the standard therapy for over 30 years and greatly improves survival among patients with AAV.[37] Current research is focused on improving efficacy and reducing side effects of the medications used to induce remission [Table 2].

One large randomized noninferiority trial of 197 patients with AAV has found Rituximab® (RTX) to be noninferior to cyclophosphamide as induction therapy,[38] including among the 102 patients with renal involvement at enrollment.[39] This finding has also been demonstrated in a separate randomized noninferiority trial of 44 patients with AAV and renal involvement.[40] Adverse events at 6 months and 1 year were not reduced in the RTX group, which had a higher number of cancers at 1 year, although this difference did not reach statistical significance.[38] Both trials were conducted in the context of case series and retrospective observational studies suggesting that RTX is efficacious as second-line therapy in patients not adequately responding to cyclophosphamide.[4142] A prospective cohort of 10 patients with refractory PR3 positive GPA tolerated and responded to RTX, although one patient relapsed after B-cell reconstitution.[43] Most centers currently use cyclophosphamide as first-line therapy, and reserve RTX for refractory cases or for those who wish to preserve their fertility[44] [
Supplementary Table 1
Supplementary Table 1 Treatment of ANCA associated vasculitisA randomized controlled trial comparing the effectiveness of daily oral cyclophosphamide versus intravenous pulses, among a sample of 149 patients with incident AAV with renal involvement, suggested that the pulse regimen significantly reduced cumulative exposure and was not associated with any significant difference in time to remission.[45] This is consistent with a prior meta-analysis of smaller studies.[46] However, a long-term follow-up study from the same cohort showed that risk of relapse was significantly higher among those who received a pulse regimen, although mortality and renal failure were not significantly different between groups.[47] Prophylaxis should be used during combined cyclophosphamide and steroid treatment against infections, bone loss and peptic ulcer. Tuberculosis can turn fatal with the initiation of therapy and, therefore, all patients should be assessed for tuberculosis continually before commencement of treatment and during the maintenance phase for fear of reactivation or de novo infection. Primary isoniazid prophylaxis is recommended for patients with latent tuberculosis with long-term immunosuppression; however with emerging isoniazid resistance and hepatotoxicity indistinguishable from viral hepatitis the appetite for such therapy is low in the tropics.
A meta-analysis of nine randomized controlled trials, including the MEPEX trial [Table 2][48] comprising 387 patients with AAV or idiopathic rapidly progressive glomerulonephritis, suggested that plasma exchange, which involves replacing the entire plasma component of the circulatory system including circulating antibodies, provided additional benefit in reducing progression to dialysis in patients with severe renal vasculitis.[49] However, whether it improved mortality outcomes or the composite measure of mortality and renal outcomes, remains unclear.[49] PEXIVAS is a 2 × 2 factorial design open-label randomized trial that is currently being conducted to answer this question (NCT00987389).[50] Approximately, 500 patients with severe incident AAV are being recruited across multiple international centers to receive randomly either plasma exchange or no plasma exchange, and either standard or reduced glucocorticoid dosing, in addition to cyclophosphamide or RTX. This trial also attempts to evaluate whether lower dose glucocorticoids influences time to all-cause mortality or end-stage renal disease (ESRD), given the dose-related side effects of this medication.[50]
Other agents, including tacrolimus, intravenous immunoglobulin (IVIG) and anti-TNF-α compounds such as etanercept and infliximab, have previously been explored in the treatment of AAV, however the current evidence supporting their use is limited and, in light of the demonstrable efficacy of other agents, are unlikely to be adopted as first-line induction therapy.[515253] Nevertheless, there are relatively few trials that have explored the efficacy of these agents in combination therapy. Case reports of IVIG improving EGPA-associated myocarditis and heart failure have also been described.[52] If these alternative agents are to be investigated further, the risks of combining multiple immunosuppressive agents will need to be weighed against the likely benefit.
Maintenance therapy
Azathioprine (AZA) was established as the drug of choice for maintenance therapy in the CYCAZAREM trial which found introducing AZA within 3 months of inducing clinical remission did not result in more early relapses than continuing cyclophosphamide for 12 months.[54] The role of AZA as the preferred agent for maintenance therapy was reinforced by head to head trials comparing AZA to mycophenolate mofetil (MMF) and methotrexate which did not find evidence to support the use of these alternative agents.[5556] Indeed, AZA was associated with fewer relapses compared to MMF without a significant difference in serious adverse event rates.[55]
However, recent landmark studies have brought both the timing of initiating maintenance therapy, and the choice of maintenance agent under renewed scrutiny [Table 3].

Specifically, in a long-term follow-up of the CYCAZAREM trial, the AZA group was found to have a nonstatistically significant trend toward higher rates of disease relapse, ESRD and death relative to the CYC group after 5 years from time of randomization.[57] Thus, it seems that the question of optimal timing to switch to maintenance therapy remains to be established.
Furthermore, a recently published randomized controlled trial comparing AZA with RTX for maintenance therapy across 115 patients with ANCA-positive AAV (MAINRITSAN), has demonstrated the superiority of RTX at preventing major relapse at 28 months.[58] This study was conducted following a series of observational studies demonstrating the efficacy of RTX as a maintenance agent.[596061] Nevertheless, the authors of this landmark trial acknowledge some important limitations, including the lack of masking, predominance of participants with PR3-ANCA, variability of glucocorticoid use protocol across study sites, and the significance of tapering the dose of AZA between 12 and 22 months. The long-term efficacy of RTX in the maintenance of AAV remission beyond 28 months is yet to be tested with randomized trials. The RITAZAREM trial (NCT01697267), which plans to compare RTX with AZA across 160 participants with relapsing disease after RTX induction therapy over 4 years of follow-up, is currently in the recruiting phase. It is likely to provide further insight into the long-term efficacy of RTX in maintenance of AAV.[62]
The optimal duration of maintenance therapy with AZA is likewise unclear. According to one opinion paper,[63] a trial is currently underway to compare 2 years versus 4 years of AZA maintenance therapy. However, the details of this trial remain unpublished, and we have been unable to locate the trial protocol. It is possible that RITAZAREM, which will include a control arm that is treated with AZA for 4 years, may have superseded this trial.
The optimal duration of steroid therapy is also yet to be established. The TAPIR[64] trial is recruiting patients with GPA in remission to evaluate the effects of using low-dose glucocorticoids (5 mg/day of prednisone) as compared to stopping glucocorticoid treatment entirely on rates of disease relapse/disease flares. Some authors have suggested that treatment duration for treatment should be based on the protocol used in the relevant induction therapy study.[65] For example, if cyclophosphamide was used as induction therapy then a “weekly glucocorticoid reduction method with consideration for longer low-dose therapy” should be implemented; whereas if RTX was used for induction then glucocorticoid therapy should be dosed according to the RAVE trial.[38]
The role for newer biological agents in maintenance therapy is also being explored. One such agent under investigation is belimumab, a monoclonal antibody to B-cell activating factor. The Belimumab in Remission of Vasculitis trial is currently recruiting participants to test whether belimumab in combination with AZA improves the relapse-free survival compared to AZA alone (NCT01663623).[66] There is also an ongoing trial using abetacept (CTLA4-Ig) for the treatment of relapsing, non-severe, GPA (ABROGATE0029 with results due in 2018.[67]
Complications and prognosis
Treatment has dramatically improved the prognosis of patients diagnosed with AAV. The median survival from diagnosis without treatment is 5 months, whereas 88% of treated patients survive 1-year and 78% survive for 5 years.[65] Despite these gains, mortality in the 1st year is still twice as high as the background rate for individuals of the same age and gender, and remains persistently elevated by 30% in subsequent years.[65]
The predominant causes of death in the 1st year differ from those in subsequent years. In the 1st year, almost 50% of deaths are related to infection and 20% from disease related complications. In subsequent years, cardiovascular disease and malignancy have become the major drivers of death, although infection and vasculitis specific causes still contribute to 20% and 6%, respectively.[65] Of note, AAV and its treatments are known to be independent risk factors for CVD and malignancy, which represent the major causes of death in the general population. AAV has been associated with higher risk of cardiovascular disease and the metabolic syndrome independently of glucocorticoids,[68] probably owing to endothelial damage related to systemic inflammation, and cyclophosphamide is known to increase the risk of nonmelanomatous skin cancers and bladder cancer.[65] Other long-term treatment related side effect which impact negatively on quality of life include glucocorticoid induced osteoporosis and diabetes, and cyclophosphamide induced gonadotoxicity and infertility, especially among young women.[65]
Infective complications following treatment are the leading cause of death in the 1st year after diagnosis and contribute to a significant ongoing morbidity and mortality in patients diagnosed with AAV. In tropical regions it is important to exclude mycobacterial infection or malaria (as the cause of presentation) before commencing treatment, as ANCA testing may be falsely positive in these patients.[69] A wide variety of infections are described, including pneumonia, cellulitis, bacteremia and opportunistic viral and fungal infections.[65] They are more common in those with poorer renal function, longer duration of glucocorticoid use and/or severe lymphopenia;[70] however, the role of monitoring for severe leukopenia is yet to be defined in the AAV population. The risk of serious infections requiring hospitalization is directly proportional to the dose of cyclophosphamide and glucocorticoids administered. Ongoing efforts to try and optimize the dosage requirements of these medications will be critical to minimizing the burden of infection.
S. aureus bacteremia is frequently identified as a cause of death amongst AAV patients, who are also known to have a high rate of nasal colonization. There is indirect evidence from dialysis and surgical populations that eradicating colonization of S. aureus with topical mupirocin may reduce the risk of infection; however, this must be balanced with the consideration that long-term treatment is likely to induce resistance and may increase the risk of non-S. aureus infections.[71] TMP-SXT for the prevention of Pneumocystis jirovecii pneumonia during induction therapy is currently the only widely accepted medication used for antimicrobial prophylaxis among patients with AAV.[71] Interestingly, TMP-SXT has also been shown to have independent anti-relapse properties in the treatment of GPA.[35]
Severe renal involvement and diffuse alveolar hemorrhage are the major vasculitic complications of AAV and are the predominant causes of vasculitis related mortality.[72] They are also well-known markers of induction-refractory disease.[72] More recently, genetic factors, including DRB1*405 and DPB1*402 have been used to stratify individual risk of poor response to treatment, deterioration of renal function, and all-cause mortality.[73]
Renal involvement is an independent predictor of mortality and progression to ESRD,[74] with 6% of those with renal involvement progressing to ESRD.[75] The risk of progression to ESRD among MPO positive patients is approximately double that of PR3 positive patients. A requirement for dialysis at presentation carries a particularly poor prognosis; 23% die within 6 months, 29% do not regain renal function, and 15% temporarily regain renal function but require dialysis within 13–63 months.[75] Paradoxically, relapse rates among ESRD patients on regular dialysis were significantly lower compared to those with preserved renal function.[76] The authors suggest that this may be due to a therapeutic effect of dialysis itself. This concept is broadly consistent with a retrospective cohort from Australia and New Zealand, which found that, once on dialysis, survival rates are similar among those diagnosed with AAV and those who developed ESRD from other causes.[77]
Histologic analysis of renal tissue aids in predicting renal outcomes. Identification of glomerular lesions as focal, mixed, crescentic, or sclerotic GN, according to the ANCA-associated GN (AGN) histopathological classification system, predicts severity of renal function impairment at one and 5 years, with the best and worst outcomes associated with focal and sclerotic lesions respectively.[78] Histologic identification of the percentage of normal glomeruli[79] and the degree of tubulointerstitial fibrosis and atrophy[80] also predict renal survival. These observations could be studied in conjunction with the AGN classification to improve prognostication.
The prognostic studies of AAV patients with ESRD that receive renal allografts demonstrate mixed findings. Among a cohort of 35 ESRD AAV patients at Mayo Clinic that received renal allografts, 5-year death censored allograft survival and 5-year patient survival were 100% and 94%, respectively.[81] However, in a retrospective analysis of over 90 ESRD AAV patients from Australia and New Zealand that received allografts, the 10-year allograft survival rates were 50%, 62% and 70%, and 10-year patient survival rates were 68%, 85% and 83% for MPA, GPA and non-AAV ESRD populations, respectively.[77]
Severity of early disease, as measured through the Vasculitis Damage Index (VDI), may also be a useful general prognostic indicator of overall mortality.[82] From a retrospective case-control study of 100 nonfatal and 20 fatal cases of systemic vasculitis in 1993, it has been estimated that the odds of death are 2–19 times higher amongst those with a VDI score of 5 or above, relative to those with a lower damage score.[83] While this analysis was conducted across the spectrum of systemic vasculitides, subgroup analysis among those with GPA was broadly consistent with the overall findings.[83]
Relapses are more likely among patients who are positive for PR3-ANCA, have cardiovascular involvement, or lung or ear-nose-throat granulomas.[584] The role of serial titers of ANCA in predicting relapse is still unclear. One retrospective cohort study has indicated that a fourfold rise in titer is predictive of relapse and suggested that preemptively increasing immunosuppression can prevent relapse.[21] On the other hand, a recent meta-analysis found that while both a rise in ANCA and persistently positive ANCA were associated with relapse at a level of statistical significance, both findings only conferred small amounts of information regarding the likelihood of increased risk in the individual patient.[22] Some experts believe that a new ANCA positivity predicts relapse after treatment withdrawal though this is not yet based on good evidence. Interestingly, a signature transcriptional profile in CD8 positive T-cells may also predict relapse in AAV,[85] although further studies are required to evaluate the clinical utility of this novel biomarker.[11] Given the therapeutic importance of detecting and treating disease recurrence, devising an algorithm to predict relapse is an important issue that warrants further investigation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 2012 revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1-11.
- [Google Scholar]
- Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007;66:222-7.
- [Google Scholar]
- Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143:621-31.
- [Google Scholar]
- Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: A cluster analysis. Ann Rheum Dis. 2013;72:1003-10.
- [Google Scholar]
- ANCA vasculitis: To lump or split? Why we should study MPA and GPA separately. Rheumatology (Oxford). 2012;51:2115-7.
- [Google Scholar]
- Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine (Baltimore). 1999;78:26-37.
- [Google Scholar]
- ACR/EULAR-endorsed study to develop diagnostic and classification criteria for vasculitis (DCVAS) Clin Exp Nephrol. 2013;17:619-21.
- [Google Scholar]
- Antineutrophil cytoplasmic antibodies and associated diseases: A review of the clinical and laboratory features. Kidney Int. 2000;57:846-62.
- [Google Scholar]
- ANCA-positive and ANCA-negative phenotypes of eosinophilic granulomatosis with polyangiitis (EGPA): Outcome and long-term follow-up of 50 patients from a single Polish center. Clin Exp Rheumatol. 2014;32(3)Clin Exp Rheumatol. 2014;32(Suppl 82):S41-7.
- [Google Scholar]
- Comparison of the epidemiology of anti-neutrophil cytoplasmic antibody-associated vasculitis between Japan and the U. K. Rheumatology (Oxford). 2011;50:1916-20.
- [Google Scholar]
- Epidemiology of ANCA-associated vasculitis. Rheum Dis Clin North Am. 2010;36:447-61.
- [Google Scholar]
- Renal vasculitis in Japan and the UK – Are there differences in epidemiology and clinical phenotype? Nephrol Dial Transplant. 2008;23:3928-31.
- [Google Scholar]
- Epidemiology of vasculitides: Differences between Japan, Europe and North America. Clin Exp Nephrol. 2013;17:611-4.
- [Google Scholar]
- Spectrum of anti-neutrophil cytoplasmic antibodies in patients with pulmonary tuberculosis overlaps with that of Wegener's granulomatosis. Indian J Med Sci. 2004;58:283-8.
- [Google Scholar]
- The Pattern of ANCA Associated Vasculitis in India. Paper Presented at: Rheumatology; 2001.
- [Google Scholar]
- Autoantibodies against neutrophils and monocytes: Tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;1:425-9.
- [Google Scholar]
- Hypotheses on the etiology of antineutrophil cytoplasmic autoantibody associated vasculitis: The cause is hidden, but the result is known. Clin J Am Soc Nephrol. 2008;3:237-52.
- [Google Scholar]
- Serial ANCA titers: Useful tool for prevention of relapses in ANCA-associated vasculitis. Kidney Int. 2003;63:1079-85.
- [Google Scholar]
- Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis – A meta-analysis. Rheumatology (Oxford). 2012;51:100-9.
- [Google Scholar]
- Complement is crucial in the pathogenesis of ANCA-associated vasculitis. Kidney Int. 2013;83:16-8.
- [Google Scholar]
- Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int. 2013;83:129-37.
- [Google Scholar]
- Oral C5a receptor antagonist CCX168 phase 2 clinical TRIAL in ANCA-associated renal vasculitis. Ann Rheum Dis. 2014;73(Suppl 2):148. OP0227(Abstract)
- [Google Scholar]
- Natural autoantibodies to myeloperoxidase, proteinase 3, and the glomerular basement membrane are present in normal individuals. Kidney Int. 2010;78:590-7.
- [Google Scholar]
- Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro . Proc Natl Acad Sci U S A. 1990;87:4115-9.
- [Google Scholar]
- Autoantibodies to hLAMP-2 in ANCA-negative pauci-immune focal necrotizing GN. J Am Soc Nephrol. 2014;25:455-63.
- [Google Scholar]
- Risks and relative risks of Wegener's granulomatosis among close relatives of patients with the disease. Arthritis Rheum. 2008;58:302-7.
- [Google Scholar]
- The contribution of genetic variation and infection to the pathogenesis of ANCA-associated systemic vasculitis. Arthritis Res Ther. 2010;12:202.
- [Google Scholar]
- Pathogenesis of anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Clin Exp Immunol. 2011;164(Suppl 1):23-6.
- [Google Scholar]
- Occupational exposure in ANCA-positive patients: A case-control study. Kidney Int. 2005;67:1961-6.
- [Google Scholar]
- Silica exposure in anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and lupus nephritis. J Am Soc Nephrol. 2001;12:134-42.
- [Google Scholar]
- Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. 1994;120:12-7.
- [Google Scholar]
- Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener's granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N Engl J Med. 1996;335:16-20.
- [Google Scholar]
- Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med. 2008;14:1088-96.
- [Google Scholar]
- Cyclophosphamide therapy of severe systemic necrotizing vasculitis. N Engl J Med. 1979;301:235-8.
- [Google Scholar]
- Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221-32.
- [Google Scholar]
- Rituximab versus cyclophosphamide for ANCA-associated vasculitis with renal involvement. J Am Soc Nephrol. 2015;26:976-85.
- [Google Scholar]
- Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211-20.
- [Google Scholar]
- A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2009;60:2156-68.
- [Google Scholar]
- Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52:262-8.
- [Google Scholar]
- Rituximab for refractory Wegener's granulomatosis: Report of a prospective, open-label pilot trial. Am J Respir Crit Care Med. 2006;173:180-7.
- [Google Scholar]
- Con: Should all patients with anti-neutrophil cytoplasmic antibody-associated vasculitis be primarily treated with rituximab? Nephrol Dial Transplant. 2015;30:1075-81.
- [Google Scholar]
- Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: A randomized trial. Ann Intern Med. 2009;150:670-80.
- [Google Scholar]
- The value of pulse cyclophosphamide in ANCA-associated vasculitis: Meta-analysis and critical review. Nephrol Dial Transplant. 2001;16:2018-27.
- [Google Scholar]
- Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: Long-term follow-up. Ann Rheum Dis. 2012;71:955-60.
- [Google Scholar]
- Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180-8.
- [Google Scholar]
- Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulonephritis: A meta-analysis. Am J Kidney Dis. 2011;57:566-74.
- [Google Scholar]
- Plasma exchange and glucocorticoid dosing in the treatment of anti-neutrophil cytoplasm antibody associated vasculitis (PEXIVAS): Protocol for a randomized controlled trial. Trials. 2013;14:73.
- [Google Scholar]
- Successful induction of granulomatosis with polyangiitis with tacrolimus. Indian J Nephrol. 2015;25:46-9.
- [Google Scholar]
- A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36-44.
- [Google Scholar]
- Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: A randomized controlled trial. JAMA. 2010;304:2381-8.
- [Google Scholar]
- Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med. 2008;359:2790-803.
- [Google Scholar]
- Long-term follow-up of cyclophosphamide compared with azathioprine for initial maintenance therapy in ANCA-associated vasculitis. Clin J Am Soc Nephrol. 2014;9:1571-6.
- [Google Scholar]
- Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371:1771-80.
- [Google Scholar]
- Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener's): Ten-year experience at a single center. Arthritis Rheum. 2012;64:3770-8.
- [Google Scholar]
- Rituximab as maintenance therapy for anti-neutrophil cytoplasmic antibody-associated vasculitis. Clin J Am Soc Nephrol. 2010;5:1394-400.
- [Google Scholar]
- Rituximab for remission maintenance in relapsing antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64:3760-9.
- [Google Scholar]
- An international, open label, randomised controlled trial comparing rituximab with azathioprine as maintenance therapy in relapsing ANCA-associated vasculitis (RITAZAREM) La Presse Médicale. 2013;42:768.
- [Google Scholar]
- Maintenance of clinical remission in ANCA-associated vasculitis. Nat Rev Rheumatol. 2013;9:127-32.
- [Google Scholar]
- Rare Diseases Clinical Research Network. The Assessment of Prednisone In Remission (TAPIR) Trial. 2015. Available from: http://www.rarediseasesnetwork.org/vcrc/research/5526.htm
- [Google Scholar]
- Complications of long-term therapy for ANCA-associated systemic vasculitis. Nat Rev Nephrol. 2012;8:523-32.
- [Google Scholar]
- US National Institutes of Health. Belimumab in Remission of VASculitis (BREVAS) Available from: https://clinicaltrials.gov/ct2/show/NCT01663623
- [Google Scholar]
- US National Institutes of Health. Abatacept for the Treatment of Relapsing, Non-Severe, Granulomatosis With Polyangiitis. 2015. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02108860
- [Google Scholar]
- Metabolic syndrome in ANCA-associated vasculitis. Rheumatology (Oxford). 2013;52:197-203.
- [Google Scholar]
- Background noise of infection for using ANCA as a diagnostic tool for vasculitis in tropical and developing countries. Parasitol Res. 2008;102:1093-5.
- [Google Scholar]
- Lymphopenia and treatment-related infectious complications in ANCA-associated vasculitis. Clin J Am Soc Nephrol. 2013;8:416-23.
- [Google Scholar]
- Value of anti-infective chemoprophylaxis in primary systemic vasculitis: What is the evidence? Arthritis Res Ther. 2009;11:253.
- [Google Scholar]
- Diffuse alveolar haemorrhage in ANCA-associated vasculitis. Intern Med. 2013;52:5-13.
- [Google Scholar]
- Association of HLA genes with clinical outcomes of ANCA-associated vasculitis. Clin J Am Soc Nephrol. 2012;7:1293-9.
- [Google Scholar]
- Renal histology in pauci-immune rapidly progressive glomerulonephritis: 8-year retrospective study. Indian J Pathol Microbiol. 2012;55:28-32.
- [Google Scholar]
- Renal survival in proteinase 3 and myeloperoxidase ANCA-associated systemic vasculitis. Clin J Am Soc Nephrol. 2013;8:1709-17.
- [Google Scholar]
- The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int. 2009;76:644-51.
- [Google Scholar]
- The outcomes of patients with ESRD and ANCA-associated vasculitis in Australia and New Zealand. Clin J Am Soc Nephrol. 2013;8:773-80.
- [Google Scholar]
- Pathological classification of anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. Clin Exp Immunol. 2011;164(Suppl 1):14-6.
- [Google Scholar]
- Estimating renal survival using the ANCA-associated GN classification. J Am Soc Nephrol. 2013;24:1371-5.
- [Google Scholar]
- ANCA serotype and histopathological classification for the prediction of renal outcome in ANCA-associated glomerulonephritis. Nephrol Dial Transplant. 2014;29:1764-9.
- [Google Scholar]
- Recurrence of ANCA-associated vasculitis following renal transplantation in the modern era of immunosupression. Kidney Int. 2007;71:1296-301.
- [Google Scholar]
- Damage assessment in ANCA-associated vasculitis. Curr Rheumatol Rep. 2012;14:494-500.
- [Google Scholar]
- Examination of disease severity in systemic vasculitis from the novel perspective of damage using the Vasculitis Damage Index (VDI) Br J Rheumatol. 1998;37:57-63.
- [Google Scholar]
- Treatment of antineutrophil cytoplasmic antibody-associated vasculitis. Curr Opin Pulm Med. 2012;18:447-54.
- [Google Scholar]
- A CD8+T cell transcription signature predicts prognosis in autoimmune disease. Nat Med. 2010;16:586-91.
- [Google Scholar]







