Translate this page into:
Renal Salt-Wasting Syndrome Induced by Neoadjuvant Chemotherapy Containing Cisplatin – A Case Report
Address for correspondence: Dr. A. Vimala, Senior Consultant, Department of Nephrology, Cosmopolitan Hospital, Thiruvananthapuram, Kerala, India. E-mail: vimala50@rediffmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hyponatremia is one of the most common electrolyte abnormality seen in oncology practice. The underlying pathogenetic mechanism for chemotherapy-induced hyponatremia is renal salt-wasting syndrome (RSWS) and syndrome of inappropriate antidiuretic hormone secretion (SIADH). Fluid restriction is the treatment of choice in SIADH, whereas salt supplements is the mode of treatment in RSWS. Hence, differentiation between RSWS and SIADH is very important though difficult. Case reports of cisplatin (cis-dichloro-diammine-platinum-2)-induced RSWS and SIADH are rare in the literature. We report about a patient who developed hyponatremia, hypokalemia with excessive urinary excretion of sodium and potassium, renal glycosuria, and aminoaciduria on the third day of the first cycle of cisplatin-containing chemotherapy.
Keywords
Cisplatin
hyponatremia
RSWS
SIADH
Introduction
Hyponatremia is very commonly seen in hospitalized patients. Patientsdevelop hyponatremia due to various reasons including gastro intestinal loss, excessive intake of water, adrenocortical insufficiency, cardiac failure, thyroid disorders, diuretics, SIADH and RSWS. Hyponatremia in patients on chemotherapy is usually attributed to SIADH. In this case report we would like to highlight the rare syndrome of renal salt wasting due to cisplatin.
Case Report
A 61-year-old man, professor by occupation, was referred to us on June 22, 2019, for the management of hyponatremic encephalopathy. The patient was referred with the following details and was diagnosed with carcinoma pyriform sinus (T4aN1M0) 2 weeks back. The patient was explained various therapeutic options, including surgery, neoadjuvant chemotherapy, and radiation in the oncology center. Since the patient insisted on conservation of voice, neoadjuvant chemotherapy was planned. The doses of chemotherapeutic agents were 75 mg/m2 of cisplatin (cis-dichloro-diammine-platinum-2) and 750 mg/m2 of 5-fluorouracil. At the time of initiation of therapy, the patient had a normal hematological (except Hb of 11 g/dL) and biochemical profile (creatinine 0.8 mg%, Na and K 134 and 4 meq/L, respectively). His calculated estimated glomerular filtration rate (eGFR) was 107 mL/min/1.73 m2.
On the third day of the first cycle of chemotherapy, the patient developed vomiting and hiccups. He was euvolemic and normotensive (blood pressure [BP] 130/70 mmHg) Systemic examination was unremarkable. He was documented to have hyponatremia (Na 112 meq/L), hypokalemia (K 2.8 meq/L) with low plasma osmolality (212 mosm/L), and high urine osmolality (431 mosm/L).
Possibility of SIADH was considered. Three percent saline was infused daily with fluid restriction. On the eighth day of chemotherapy, the patient developed features of encephalopathy as evidenced by the alteration of sensorium and slurring of speech. Despite administration of 3% saline for 3 consecutive days, his serum sodium dropped to 101 meq/L and sensorium deteriorated. Other electrolyte abnormalities included hypokalemia (K 2.1 meq/L), hypocalcemia (Ca 7.6 mg%), and mild hypomagnesemia (Mg 1.7 mg%). His blood urea and serum creatinine were within normal limits. Urine volume was not mentioned.
He was referred to the nephrology department for further management.
On examination, the patient was stuporous and had a pulse rate 62/min, BP 150/90 mmHg in lying down position, SpO298% in room air with no edema, normal skin turgor, and no evidence of fluid overload.
He had slurring of speech with no focal neurological deficit. Systemic examination was otherwise unremarkable. His weight was 55 kg.
He had severe hyponatremia with sodium of 98 meq/L, hypokalemia with potassium of 2.19 meq/L, and corrected calcium of 8.4 mg/dL. He had low plasma osmolality (211 mosm/L) and high urine osmolality (482 mosm/L) with urine sodium of 130 meq/L and urine potassium of 30 meq/L.
His urine output was around 150 to 400 mL/hour with a 24-hour urinary volume of 3,000 mL. His blood gas analysis showed respiratory alkalosis.
Cortisol deficiency and hypothyroidism was excluded by plasma cortisol estimation (4 p.m. cortisol 19.3 μg/dL and morning cortisol 15.8 μg/dL) and thyroid function tests (TSH [thyroid stimulating hormone] 0.92 mIU/L). His eGFR was normal throughout the hospital stay.
In this patient with high urine sodium and high urine osmolality, the possibilities were SIADH (syndrome of inappropriate anti diuretic hormone secretion) and renal salt wasting syndrome (RSWS). Polyuria with associated hypokalemia, potassium wasting in the urine, hypocalcemia (Ca 8.4 mg%), hypomagnesemia (Mg 1.7 mg%), renal glycosuria (URE Ph 7.5, glucose 70 [green], RBCs [red blood cells] 20–22, pus cells 4–6), and aminoaciduria (urine for amino acids leucine and valine present) were diagnostic of RSWS and proximal tubular injury. Failure to correct hyponatremia with fluid restriction alone in the primary hospital was against the diagnosis of SIADH. So the final diagnosis was proximal tubular injury leading to RSWS, which had resulted in various electrolyte abnormalities, mainly severe hyponatremia. The reference letter showed only 5-fluorouracil in addition to cisplatin. We could not get any reference showing 5-fluorouracil–induced hyponatremia, hence cisplatin was considered as the offending agent.
The management strategy employed was to raise the sodium by 5 meq/L using 3% saline replacing other electrolytes with volume correction.
Sodium requirement was calculated based on the deficit of sodium and potassium, maintenance requirement, and urinary loss of sodium. On the first day of admission, the patient was given 700 mL of 3% saline and 20 meq of potassium chloride (KCl) infusion along with 15 g of sodium chloride and 80 meq of KCl through the Ryle's tube. His serum sodium increased to 108 meq/L and potassium to 2.4 meq/L. On the second day of admission, he received 600 mL of hypertonic saline, 15 g of salt, and 80 meq of KCl through the Ryle's tube. His serum sodium increased to 116 meq/L and potassium to 3.6 meq/L. Correction of hyponatremia was continued as above. After 96 hours of admission, his serum sodium increased to 123 meq/L and potassium to 3.7 meq/L. His serum calcium and magnesium normalized. His sensorium became normal, and speech was normal. Clinical improvement and normalization of electrolytes with salt and potassium supplements along with fluids confirmed the diagnosis of RSWS. He was discharged with advice to continue salt and potassium supplements. The algorithmic approach to hyponatremia is shown in Figure 1 with reference to this patient.
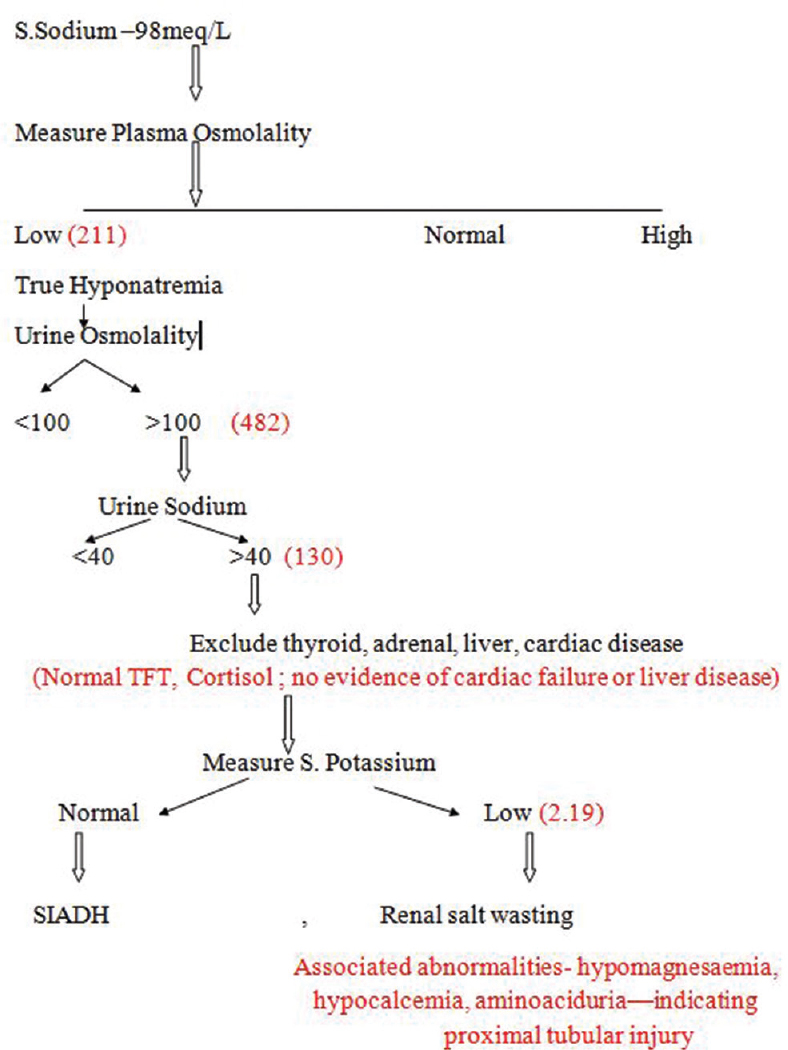
- Algorithm showing approach to hyponatremia with reference to this study's patient
Discussion
Although hyponatremia is a common electrolyte abnormality encountered in patients with malignancy, RSWS as a cause of hyponatremia is rare. The disease itself or the chemotherapeutic agents may be responsible for hyponatremia. The incidence in hospitalized population varies from less than 1% to more than 40%.[1] Hyponatremia following cisplatin chemotherapy was reported in 1989 by Bisset et al. Vinca alkaloids, platinum compound, alkylating agents, and immunomodulators are common therapeutic agents known to induce hyponatremia.[2] The incidence of hyponatremia in the first cycle of chemotherapy was 64.5%. Our patient also developed hyponatremia in the first cycle. The usual risk factors mentioned are bodyweight <60 kg, creatinine clearance <60 mL/min/1.73 m2, sodium depletion, and decreased fluid intake due to treatment-induced anorexia, nausea, vomiting, and diarrhea.[3] Risk factors in our patient were bodyweight of 55 kg and vomiting. His eGFR was 107 mL/min/1.73 m2, and his sodium intake was normal. There are two mechanisms by which cisplatin can induce hyponatremia SIADH (syndrome of inappropriate antidiuretic hormone secretion) and RSWS (renal salt wasting syndrome).[4] Differential diagnosis of hyponatremia induced by cisplatin was discussed by Mari Inamori in 2016.[5] Both SIADH and RSWS have many common laboratory abnormalities. High urine sodium loss with low plasma osmolality and high urine osmolality are seen in both situations. Hypokalemia with increased urinary potassium loss, renal glycosuria, aminoaciduria, and polyuria point toward RSWS.[5] Cisplatin exerts its damage on different segments of nephron. Decreased GFR results in elevation of blood urea and serum creatinine and damage to the proximal tubules, the major sites of water reabsorption results in RSWS. The consequences are increased urinary sodium loss and hypovolumia with increased urine output and urine sodium. The restoration of volume results in the correction of abnormalities.[6]
Our patient had polynatriuresis, hypokalemia, hypocalcemia, and hypomagnesemia, which gave clue to the diagnosis of RSWS. The patient was managed with restoration of volume status and salt administration.
To summarize, cisplatin, one of the most widely used chemotherapeutic agent, is well-known to produce renal impairment with decline in GFR, but RSWS and SIADH are rare. Only 17 case reports of cisplatin-induced RSWS are available in the literature. Because of the similarities in clinical settings and investigations, it is often misdiagnosed as SIADH. The differential diagnosis of RSWS due to proximal tubular injury from SIADH is very important because of the difference in therapeutic modality.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Hyponatremia related to medical anticancer treatment. Support Care Cancer. 1996;4:341-50.
- [Google Scholar]
- Hyponatremia with cisplatin administration in head and neck cancer patients. Gan To Kagaku Ryoho. 2010;37:2861-5.
- [Google Scholar]
- Renal salt wasting in patients treated with high-dose cisplatin, etoposide, and mitomycin in patients with advanced non-small cell lung cancer. Korean J Intern Med. 1992;7:118-21.
- [Google Scholar]
- Differential diagnosis of hyponatremia induced by cisplatin-containing chemotherapy: Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) or renal salt wasting syndrome (RSWS) Acta Oto-Laryngologica Case Reports. 2016;1(1):33-35. DOI: 10.1080/23772484.2016.1198231
- [Google Scholar]







