Translate this page into:
Retrieval of kidney tissue for light microscopy from frozen tissue processed for immunofluorescence: A simple procedure to avoid repeat kidney biopsies
Address for correspondence: Dr. Vineeta V. Batra, Department of Pathology, Academic Block, G B Pant Hospital, Jawaharlal Nehru Marg, New Delhi - 110 002, India. E-mail: vvbatra9@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We highlight a method that is helpful in situations where the tissue sent for LM is inadequate whereas the tissue sent for IF showed glomeruli useful for interpretation. We utilized the leftover frozen tissue after the sections for IF were taken. This tissue was post-fixed in formalin for the purpose of light microscopic diagnosis. The glomerular pathology could be commented upon with a fair degree of accuracy and a repeat biopsy was avoided in 74.7% of the cases. However, the tubules showed marked fixation artefact and tubular pathology was distorted. This procedure can help to reach a correct diagnosis in large percentage of cases otherwise labeled as inadequate biopsy and hence, save the patient from the trauma of a repeat biopsy.
Keywords
Frozen tissue
histopathology
immunofluorescence
inadequate sample
renal biopsy
Introduction
Renal biopsy is a principal diagnostic method used by the nephrologist in the study of renal diseases. Kidney biopsy is examined using light microscopy (LM), immunofluorescence (IF), and electron microscopy. Adequate tissue must be available for all the three techniques for a full and final answer to the clinical problem.
Despite improvement in biopsy techniques, many a times tissues sent for LM or IF may show insufficient number of glomeruli for diagnosis.
It is a common practice to obtain one core and divide it into two for LM and IF. As a result, the IF or LM tissue may have only medulla.
In situations where the tissue for IF shows cortical tissue, while the tissue for LM shows only medulla, a repeat biopsy for LM might be needed. To overcome this problem, we practice a procedure of recovering tissue for LM from the IF tissue once adequate number of frozen sections are obtained.
Methods
This retrospective study was performed at the Department of Pathology, GB Pant Hospital between 2003 and 2011. Two cores of renal tissue were usually received, one in buffered formalin for LM and the other in Michels’ medium[1] for IF. Instead of simultaneously processing both the tissues, the tissue sample sent for LM was first processed and slides prepared were stained with Haematoxylin and eosin (H&E), stain to look for the presence of cortical tissue. If the tissue was adequate, i.e., a minimum of seven glomeruli were present, it was processed for LM, but if the tissue had insufficient number of glomeruli, the sample sent for IF was processed with care and salvaged as much as possible.
For frozen sectioning, the tissue was washed three times for 10 min in wash buffer prepared by standard method.[1] The tissue was then rapidly frozen at −25°C and sectioned at 3 μ thickness. The sections were stained with H and E to identify cortical tissue. Sections were picked up as soon as cortical tissue was identified rather than cutting deeper till full face of the tissue was exposed. Routinely we pick up two sections per slide and cut about 20 slides upfront.
In cases where the tissue for LM was inadequate, as soon as even one glomerulus was identified, sections were picked up for IF. Also, only one section was picked up per slide. About 15 such sections were prepared upfront and kept in the refrigerator for further processing. The remaining block with tissue was washed in wash buffer and the tissue was put in formalin for secondary fixation.
This tissue was processed and embedded in paraffin. Sections were stained with H and E and other special stains as usual.
Results
We received a total of 1,094 renal biopsies during the study perios. A total of 87 [7.95%] were inadequate on LM but the tissue sent for IF showed renal cortex. In 69 (79.3%) cases, adequate material could be retrieved and a diagnosis made [Table 1]. In 18 cases (20.7%), the tissue retrieved after frozen sectioning was insufficient for diagnosis.
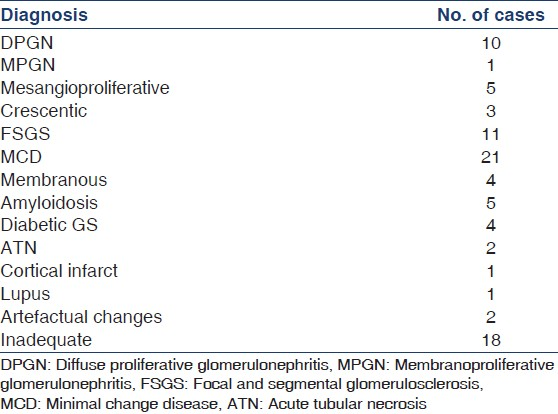
Of the 69 cases, a definite diagnosis could be offered in 65 cases. In the remaining four cases, a marked freezing artefact was seen producing morphological artefacts and a definitive diagnosis was not possible. These included two cases of acute tubular necrosis. Thus, by this method we could avoid a repeat biopsy in 74.7% of the inadequate biopsies.
The tissue so retrieved showed freezing-melting artefacts. However, the tissue was such that the glomerular pathology could be commented upon with fair degree of accuracy. Cases of diffuse proliferative glomerulonephritis (DPGN) and membranoproliferative glomerulonephritis (MPGN) could be diagnosed because of enlarged size of glomeruli and hypercellularity. Splitting of basement membrane was noted in cases of MPGN on Silver methenamine (SM) stain. In addition the IF findings in both these groups were characteristic. Thus LM and IF findings jointly helped in arriving at a definitive diagnosis. Lupus nephritis with presence of wire loop lesions, hyaline thrombi, and necrotizing lesions could be commented upon.
Crescents were also easily diagnosed. Spikes were identified in cases of membranous glomerulonephritis (MGN). The IF pattern in cases of MGN was also classical and helped to confirm the diagnosis. Amyloid deposits could be confirmed on special stains like Congo red under polarized microscope. Nodular glomerulosclerosis suggestive of diabetic nephropathy was corroborated with the clinical history. Cases of focal and segmental glomerulosclerosis could also be diagnosed, though with some difficulty.
However, the tubular pathology showed fair amount of freezing-melting artefact. Most of the tubules showed damage to the tubular epithelium and crush effect. Thus acute tubular injury could not be interpreted easily. The interstitium showed a glassy appearance and it was also difficult to comment upon the presence of chronic parenchymal damage. In cases of lupus nephritis, comments on activity and chronicity were also not possible.
A panel of photomicrographs of processed IF tissue, corresponding histochemical stains along with photomicrographs of formalin fixed tissues to compare the variation in morphology have been depicted in Figures 1–4.
![(a) Photomicrograph of formalin fixed tissue in a patient of minimal change disease [H and E, ×400]; (b) Photomicrograph of processed IF tissue [H and E, ×400]; (c) Jones’s silver stain of the case shown in B displaying unremarkable basement membrane [×200]; (d) Photomicrograph of formalin fixed tissue in a patient of FSGS [PAS stain ×400]; (e) Photomicrograph of processed IF tissue showing segmental sclerosis in the glomerular tuft, indicated by arrow [H and E, ×400]; (f) same highlighted by MT stain [×400]](/content/170/2013/23/3/img/IJN-23-206-g002.png)
- (a) Photomicrograph of formalin fixed tissue in a patient of minimal change disease [H and E, ×400]; (b) Photomicrograph of processed IF tissue [H and E, ×400]; (c) Jones’s silver stain of the case shown in B displaying unremarkable basement membrane [×200]; (d) Photomicrograph of formalin fixed tissue in a patient of FSGS [PAS stain ×400]; (e) Photomicrograph of processed IF tissue showing segmental sclerosis in the glomerular tuft, indicated by arrow [H and E, ×400]; (f) same highlighted by MT stain [×400]
![(a) Photomicrograph of formalin fixed tissue in a patient of membranous glomerulonephritis displaying thickened basement membranes [PAS stain, ×400]; (b) Photomicrograph of processed IF tissue [H and E, ×200]; (c) Case shown in B with spikes being appreciated (arrow) [SM stain ×400]; (d) Photomicrograph of formalin fixed tissue in a patient of crescentic glomerulonephritis, with cellular crescent [H and E, ×200]; (e) Photomicrograph of processed IF tissue with fibrocellular crescent indicated by arrow [H and E, ×200]; (f) crescent better highlighted on PAS stain [×200]](/content/170/2013/23/3/img/IJN-23-206-g003.png)
- (a) Photomicrograph of formalin fixed tissue in a patient of membranous glomerulonephritis displaying thickened basement membranes [PAS stain, ×400]; (b) Photomicrograph of processed IF tissue [H and E, ×200]; (c) Case shown in B with spikes being appreciated (arrow) [SM stain ×400]; (d) Photomicrograph of formalin fixed tissue in a patient of crescentic glomerulonephritis, with cellular crescent [H and E, ×200]; (e) Photomicrograph of processed IF tissue with fibrocellular crescent indicated by arrow [H and E, ×200]; (f) crescent better highlighted on PAS stain [×200]
![(a) Photomicrograph of formalin fixed tissue of a case of cortical infarct [H and E, ×100]; (b) and (c) Photomicrograph of processed IF tissue of a case of cortical infarct showing necrosed glomeruli (arrows) [H and E & PAS stain, ×100]; (d) Photomicrograph of formalin fixed tissue of a case of diabetic nephropathy [PAS stain ×200]; (e) Photomicrograph of processed IF tissue showing nodular glomerulosclerosis [KW lesion (arrow)] [PAS stain ×200]; (f) Photomicrograph of mesangioproliferative glomerulonephritis displaying increase in mesangial matrix and cellularity [H and E, ×400]](/content/170/2013/23/3/img/IJN-23-206-g004.png)
- (a) Photomicrograph of formalin fixed tissue of a case of cortical infarct [H and E, ×100]; (b) and (c) Photomicrograph of processed IF tissue of a case of cortical infarct showing necrosed glomeruli (arrows) [H and E & PAS stain, ×100]; (d) Photomicrograph of formalin fixed tissue of a case of diabetic nephropathy [PAS stain ×200]; (e) Photomicrograph of processed IF tissue showing nodular glomerulosclerosis [KW lesion (arrow)] [PAS stain ×200]; (f) Photomicrograph of mesangioproliferative glomerulonephritis displaying increase in mesangial matrix and cellularity [H and E, ×400]
![(a) Photomicrograph of formalin fixed tissue in a case of amyloidosis showing deposition of eosinophilic material along glomerular capillary loops [H and E, ×200]; (b) Photomicrograph of processed IF tissue showing amyloidosis (arrow) [H and E, ×400]; (c) Amyloid showing apple green birefringence under polarized light; (d) Photomicrograph of formalin fixed tissue showing features of acute tubular necrosis (arrow) [H and E, ×100]; (e) and (f) Photomicrographs showing poor tubular morphology and degenerative changes in tubular epithelial cells [H&E & PAS stains respectively, ×200]](/content/170/2013/23/3/img/IJN-23-206-g005.png)
- (a) Photomicrograph of formalin fixed tissue in a case of amyloidosis showing deposition of eosinophilic material along glomerular capillary loops [H and E, ×200]; (b) Photomicrograph of processed IF tissue showing amyloidosis (arrow) [H and E, ×400]; (c) Amyloid showing apple green birefringence under polarized light; (d) Photomicrograph of formalin fixed tissue showing features of acute tubular necrosis (arrow) [H and E, ×100]; (e) and (f) Photomicrographs showing poor tubular morphology and degenerative changes in tubular epithelial cells [H&E & PAS stains respectively, ×200]
Discussion
Performing renal biopsy is a hazardous procedure in the best of hands involving a fair degree of morbidity and mortality varying from 2.4% to 23.7% in different centers.[2–4] International guidelines have been fixed on the processing and evaluation of kidney biopsies to enable proper interpretation of the findings.[56] A repeat kidney biopsy is an unpleasant procedure.
By the above described method, we could avoid a repeat in 74.7% of inadequate biopsies. This method has been documented earlier with a high rate of success.[7]
These IF samples so processed in formalin tend to show freezing-melting artefact. The histomorphology is poorly preserved in comparison with that observed in a tissue fixed in buffered formalin as soon as it is biopsied. However, the glomerular pathology can be commented upon with a fair degree of accuracy thus avoiding a repeat biopsy.
As seen in this study, we could diagnose cases of DPGN, MPGN and minimal change disease (MCD). However, due to fixation artefact, tubular pathology and acute tubular necrosis could not be interpreted properly. Degree of chronic parenchymal damage also could not be commented upon due to marked fixation artefact in the tubulo-interstitial compartment. In addition, the degree of activity and chronicity in lupus nephritis could not be commented upon.
The IF tissue can be evaluated only after the LM tissue is processed, resulting in a delay in the processing of the IF tissue by 24 h. However, this small delay could be acceptable in comparison with a repeat invasive kidney biopsy.
In some centers a small portion of the cortex from the single core biopsy is processed for IF before fixing the remaining core in formalin, with a view to avoid taking two cores of tissue and decrease the incidence of complications.[8] However, in our experience, often this small bit of cortical tissue for IF is difficult to handle, especially by a poorly trained technician. On the other hand, processing two separate cores may yield adequate tissue for IF, which might provide accurate insight into the primary etiology of the disease and subsequent treatment. Moreover it may be helpful in instances when the formalin fixed tissue is inadequate for interpretation and the IF tissue may be require processing in formalin for more definite opinion.
Conclusion
In despite the degree of fixation artefact obtained when retrieving IF tissue for LM, the quality of tissue was such that the glomerular pathology could be identified in large percentage (74.7%) of cases and a repeat kidney biopsy had been avoided. This process may be tried and might avoid the trauma of a repeat biopsy.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Preservation of tissue-fixed immunoglobulins in skin biopsies of patients with lupus erythematosus and bullous diseases - Preliminary report. J Invest Dermatol. 1972;59:449-52.
- [Google Scholar]
- Percutaneous renal biopsy of native kidneys: Efficiency, safety and risk factors associated with major complications. Arch Med Sci. 2011;7:823-31.
- [Google Scholar]
- Percutaneous ultrasound-guided renal biopsy: A Libyan experience. Indian J Nephrol. 2010;20:76-9.
- [Google Scholar]
- Safety profile of paediatric percutaneous ultrasonography-guided renal biopsies. Singapore Med J. 2010;51:481-3.
- [Google Scholar]
- A simple method for utilizing frozen bioptical kidney tissue for light microscopy. Am J Nephrol. 1987;7:328-9.
- [Google Scholar]
- Routine immunofluorescence and light microscopy processing with a single renal biopsy specimen: 18 years’ experience in a single centre. J Nephrol. 2000;13:116-9.
- [Google Scholar]







