Translate this page into:
Rhinovirus Pneumonia, Rhabdomyolysis-Induced Acute Kidney Injury, and Post-Viral Forme Fruste Lupus
Corresponding author: Dr. Vijoy Kumar Jha, Physician and Nephrologist, Department of Nephrology, Command Hospital Air Force, Bengaluru - 560 007, Karnataka, India. E-mail: vkjhamd@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jha VK, Akal RS, Singh BP, Walia GK, Tejesh BS, Mahapatra D. Rhinovirus Pneumonia, Rhabdomyolysis-Induced Acute Kidney Injury, and Post-Viral Forme Fruste Lupus. Indian J Nephrol. 2024;34:389-92. doi: 10.4103/ijn.ijn_16_23
Abstract
Viral interstitial pneumonia is rarely associated with rhabdomyolysis-induced acute kidney injury (AKI) and evolving systemic lupus erythematosus (SLE) with no lupus flare. Here, we report an adult male with human rhinovirus-associated viral pneumonia and rhabdomyolysis-related AKI requiring dialysis. He was detected to be anti-nuclear, anti-Smith, and anti-U1 ribonucleoprotein antibodies positive. His kidney biopsy revealed normal glomeruli, with immunofluorescence showing a full-house pattern. Renal function and lung function gradually improved to normal without any immunosuppressants.
Keywords
Acute kidney injury
Rhabdomyolysis
Rhinovirus
Systemic lupus erythematosus
Introduction
Viral or bacterial infections are common precipitants for rhabdomyolysis and have been proposed to modulate the development of autoimmune diseases. Human rhinovirus infection can lead to severe pulmonary and extrapulmonary complications. Its association with rhabdomyolysis and evolving systemic lupus erythematosus (SLE) is not reported in adults. Here we report a case of an adult male who presented with human rhinovirus-associated viral pneumonia, rhabdomyolysis-related acute kidney injury (AKI), and positive autoimmune markers with full house pattern on renal biopsy.
Case Report
A 39-year-old male, hypertensive for 3 years, presented with a 2-week history of cough, breathlessness on exertion, vomiting, and reduced urine output (400–500 ml/day). There was no history of fever, chest pain, palpitations, diarrhea, dysuria, hematuria, graveluria, loin pain or history suggestive of connective tissue disorder. He was being treated at a local hospital with antihistamines and oral amoxicillin + clavulanate. His examination at presentation revealed tachycardia (110/min), accelerated hypertension (180/116 mmHg), and desaturation (SpO2 of 88% at room air). The respiratory system revealed bilateral basal crackles. The rest of the examination was normal. His investigations revealed neutrophilic leukocytosis with normal levels of other cell lines (hemoglobin [Hb]- 14.2 g%, total leukocyte count (TLC)- 19,100 cells/mm3, platelets- 4.8 × 106/µl). Urine routine/microscopic examination showed urine albumin 1+, no red blood cells (RBCs), and 5–10 pus cells/high-power field (HPF). Urine for myoglobinuria was positive. He had advanced azotemia (blood urea- 287 mg/dl and serum creatinine- 16.5 mg/dl), transaminitis (aspartate transaminase (AST)- 2946 IU/l, alanine transaminase (ALT)- 946 IU/l, total bilirubin- 1.5 mg/dl, and direct bilirubin- 0.3 mg/dl), and raised creatine phosphokinase (CPK) levels (15,000 U/l). His procalcitonin was increased (35.64 ng/ml), raising the suspicion of possible secondary bacterial sepsis. Chest X-ray revealed bilateral interstitial opacities involving all lung zones. Ultrasonography (USG) revealed normal-sized kidneys, normal liver size and echotexture.
In view of oliguric renal failure, metabolic acidosis (arterial blood gas [ABG] pH- 7.24, bicarbonate [HCO3]- 12 mEq/l, partial pressure of carbon dioxide (pCO2)- 18 mmHg), raised CPK values, and chest X-ray findings, the patient was managed as bilateral interstitial pneumonia with rhabdomyolysis-related acute kidney injury (AKI). He was started on broad-spectrum intravenous antibiotics (inj. meropenem + azithromycin + teicoplanin) in renal-modified doses, which was continued for 1 week. Inj. meropenem was continued empirically for 3 weeks due to raised procalcitonin, neutrophilic leukocytosis with a shift to the left on the peripheral blood smear, suspicion of secondary bacterial pneumonia, and multiorgan involvement. He was started on hemodialysis (HD) through the right internal jugular vein (IJV) catheter and underwent a total of nine HD sessions. His 2D-echocardiography revealed normal left ventricular (LV) function. Noncontrast computerized tomography (NCCT) chest images revealed multifocal areas of ground glass opacities with few areas of consolidation, consistent with acute interstitial pneumonia [Figure 1a]. Bronchoalveolar lavage (BAL) was normal with sterile BAL cultures. BAL multiplex polymerase chain reaction (PCR) for respiratory viral panel revealed human rhinovirus (HRV) positive. There was no evidence of diffuse alveolar hemorrhage (DAH). His general condition and urine output gradually improved, and he became dialysis independent over the next 3 weeks and had S. creatinine of 1.1 mg/dl. Tropical infections workup including malarial parasite (Slides and Card test), dengue (IgM/Ns1 Ag), Widal, scrub typhus IgM, and Leptospira IgM were negative. Serial blood and urine cultures were sterile. The S. galactomannan index was negative (0.32). His immunological workup revealed Antinuclear antibody (ANA) 2+ speckled pattern (1:100 dilution) with anti-Smith (anti-Sm) as well as anti-U1-RNP antibody positivity (done twice). Other autoimmune workup revealed that anti-dsDNA (24.99 IU/ml, Normal <30.00 IU/ml), C3- 164.30 mg/dl, C4- 50.50 mg/dl, and angiotensin-converting enzyme (ACE)- 50 U/l (9–67) levels were normal. Anti-glomerular basement membrane (anti-GBM), perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA), and cytoplasmic pattern anti-neutrophil cytoplasmic antbodies (c-ANCA) were negative. Viral markers were negative. Follow up investigations are as depicted in Table 1.
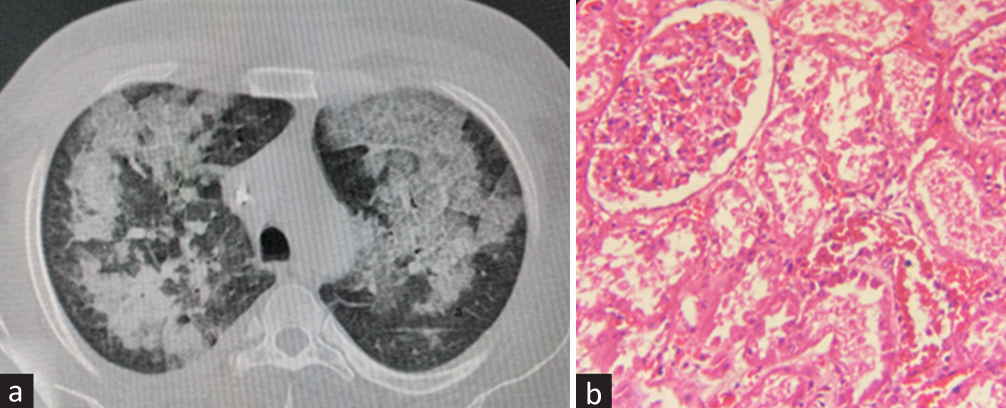
- (a) NCCT chest lung window (axial section): multifocal areas of ground glass opacities with few areas of consolidation, consistent with acute interstitial pneumonia. (b) Renal biopsy (H and E, 400×). Normal glomerulus with tubules showing acute tubular necrosis. The interstitium is edematous with few inflammatory cells. H and E = hematoxylin and eosin, NCCT = noncontrast computerized tomography.
| Investigations | Day of admission (D0) | D14 | D21 | D30 |
|---|---|---|---|---|
| Hb (g/dl)/TLC (cells/mm3)/platelets (lakhs) | 10.1/25,000/3.25 | 8.9/15,200/3.92 | 9.6/10,500 | 12.4/11,900/3.08 |
| Creatinine (mg/dl) | 16.2 | 7.06 | 1.93 | 1.10 |
| Na/K (mEq/l) | 136/3.36 | 130/4.73 | 129/3.75 | 136/4.0 |
| Ca++/phosphate (mg/dl) | 9.73/10.46 | 9.20/5.2 | 6.89/4.75 | 8.8/4.2 |
| Protein/albumin (g/dl) | 6.33/3.03 | 6.20/2.28 | 6.67/2.96 | 6.5/3.3 |
| Bil (mg/dl) | 0.85 | 0.63 | 0.6 | 1.02 |
| AST/ALT (U/l) | 51/84/ | 80/59 | 28/27 | 19/15 |
| CPK (U/l) | 15,000 | 3300 | 666 | 40 |
ALT=alanine transaminase, AST=aspartate transaminase, Bil=bilirubin, CPK=creatine phosphokinase, Hb=hemoglobin, TLC=total leukocyte count
In view of renal dysfunction and positive ANA, he was subjected to a kidney biopsy, which showed nonobsolescent 22 glomeruli. All glomeruli were normal with no evidence of segmental sclerosis, the collapse of tufts or hyperplasia of podocytes, fibrinoid necrosis, or crescents. There was moderate acute tubular injury with no interstitial fibrosis or tubular atrophy (IFTA) [Figure 1b]. Direct immunofluorescence (DIF) showed a full-house pattern, diffuse and global mesangial granular deposits with C1q (3+), IgG (3+), IgA (1+), IgM (1+), and C3 (2+). In view of ANA, anti-Sm, and anti-U1 RNP positivity and kidney biopsy suggestive of full-house pattern, the possibility of postinfectious evolving lupus was kept. He was started on hydroxychloroquine (HCQ). His repeat ANA profile after 2 months again revealed ANA 2+ speckled pattern (1:100 dilution) with anti-Sm as well as anti-U1 RNP antibody positivity, though he was asymptomatic.
Discussion
Viruses are common cause of community-acquired pneumonia (CAP). The advent of newer molecular diagnostic techniques like multiplex PCR has led to increased diagnosis of viral etiology of pneumonia compared to previous decades. HRV accounts for up to 29%–55% of viral pneumonia.1,2 Though the patient discussed here had raised procalcitonin, neutrophilic leukocytosis with shift to the left, and multiorgan involvement, accelerated hypertension pointed against secondary bacterial sepsis. HRV has been shown to cause rhabdomyolysis in pediatric settings, with two case reports showing an increase in CPK in the range of more than 30,000–50,000 IU/l. Viruses known to be associated with rhabdomyolysis include influenza A/B, parainfluenza, Epstein–Barr virus (EBV), coxsackie, herpes simplex, cytomegalovirus (CMV), and adenovirus.3 Rhinovirus-related rhabdomyolysis has been reported to date in three pediatric patients as shown in Table 2.4-6
| Reference | Age/sex | Max. S. creatinine | Max. CPK | Other organ involvement | Management |
|---|---|---|---|---|---|
| Albar et al.4 | 10 years/male | 0.3 mg/dl | 35,892 IU/l | Raised liver enzymes. No acute kidney injury | Hydration and supportive treatment |
| Tan et al.5 | 5 years 9 months/male | - | 50,741 IU/l | Raised cardiac and liver enzymes | Oxygen support, hydration, antibiotics |
| Habib et al.6 | 7 years/male | 2.5 mg/dl | 91,450 IU/l | Hypoxia/hypotension | Hemodialysis for 10 days, supportive |
| Present case | 39 years/male | 16.5 mg/dl | 15,000 IU/l | Bilateral interstitial pneumonia | Hemodialysis/noninvasive ventilation/supportive |
CPK=creatine phosphokinase
Our patient had severe interstitial pneumonia, which was proven to be HRV related (BAL multiplex PCR positive). Though lupus pneumonitis was one of the important differentials, clinical response without any immunosuppressants and normal BAL findings favor the diagnosis of viral interstitial pneumonia. He also had rhabdomyolysis (CPK >15,000 U/l) with AKI requiring hemodialysis support for more than 2 weeks. He gradually improved and became dialysis independent over the next 3 weeks, and his creatinine settled down to 1.1 mg/dl. Though he had no signs and symptoms suggestive of connective tissue disorder, he was detected to have ANA (Immunofluorescence) with anti-Sm and anti-U1 RNP antibodies positivity, generating the possibility of postinfectious evolving lupus. His kidney biopsy was also suggestive of full-house pattern in DIF, though the glomeruli appeared essentially normal with tubules, suggestive of acute tubular necrosis secondary to rhabdomyolysis. Infections can precipitate a flareup of systemic lupus erythematosus (SLE). Various viruses have been found to cause postinfectious SLE, including EBV, CMV, parvovirus B19, human endogenous retrovirus (HERV), and human immunodeficiency virus (HIV)-1, though the exact mechanism of activation is still debatable.
Viral infections affect the host immune system by superantigen production, structural or functional molecular mimicry, epitope spreading, bystander activation, altered apoptosis, clearance deficit, and innate immunity activation with type I interferon production. All of these mechanisms result in a reduction in tolerance, increased autoantibodies production, immune complexes deposition, and tissue damage.7 Various pattern recognition receptors (PRRs) promote the activation of dendritic cells and B cells after binding viral nucleic acids (NAs), which may potentiate the risk of potential autoreactivity against abundant self-NAs.7
There are many case reports on post-coronavirus disease 2019 (post-COVID-19) infection activation of SLE. Reports by Ramachandran et al.8 and Assar et al.9 have postulated the role of interferon 1, B-cell activation, and cytokine storm as the predominant mechanisms for SLE development post-COVID infection.
Bacterial and parasitic infections have also been linked with the activation of SLE like in tuberculosis, and various molecular and epidemiological data suggest an association between tuberculosis and SLE activation. The Helicobacter pylori infection may possibly have a protective role in reducing SLE activation.10
As our patient had a full-house pattern in renal biopsy without the obvious clinical features of SLE, the diagnosis of non-lupus full-house nephropathy cannot be ignored.11 The presence of ANA positivity along with anti-Sm antibody positivity highly favors evolving lupus or new-onset SLE, as anti-Sm is quite specific (>90%) for SLE but can only be seen in 5%–30% of patients and portends severe disease and development of lupus nephritis.12 The U1 RNP antibody positivity is nearly 100% sensitive for mixed connective tissue disorder (MCTD), but may be associated with anti-Sm antibody in 20%–30% of SLE cases.13
Conclusion
We report a case of viral pneumonia (HRV) with rhabdomyolysis-related AKI, ANA positivity, renal dysfunction, bilateral lung infiltrates, and mild proteinuria who presented a diagnostic dilemma between viral infection and lupus flare. In view of the features not fulfilling the classification criteria of SLE, he needs to be carefully followed up for evolving lupus. Lupus and lupus-like illness lie on a spectrum from antibody only to evolving lupus to typical lupus to overlap disease.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Rhinovirus is associated with severe adult community-acquired pneumonia in China. J Thorac Dis. 2017;9:4502-11.
- [CrossRef] [PubMed] [Google Scholar]
- Viral myositis leading to rhabdomyolysis: A case report and literature review. Am J Emerg Med. 2009;27:372.e5-6.
- [CrossRef] [PubMed] [Google Scholar]
- Rhabdomyolysis induced by rhinovirus: A case report. Cureus. 2022;14:e22784.
- [CrossRef] [Google Scholar]
- Rhabdomyolysis caused by rhinovirus. Glob Pediatr Health. 2016;6;3:2333794X16643726. doi: 10.1177/2333794X16643726
- [CrossRef] [PubMed] [Google Scholar]
- Rhinovirus-associated rhabdomyolysis and acute renal failure in a pediatric patient. Crit Care Med. 2018;46:343.
- [CrossRef] [Google Scholar]
- Viral infections and systemic lupus erythematosus: New players in an old story. Viruses. 2021;13:277.
- [CrossRef] [PubMed] [Google Scholar]
- New onset systemic lupus erythematosus after COVID-19 infection: A case report. AME Case Rep. 2022;6:14.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic lupus erythematosus after coronavirus disease-2019 (COVID-19) infection: Case-based review. Egypt Rheumatol. 2022;44:145-9.
- [CrossRef] [Google Scholar]
- Infectious processes and systemic lupus erythematosus. Immunology. 2019;158:153-60.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological pattern of non-lupus full house nephropathy. Indian J Nephrol. 2020;30:301-6.
- [Google Scholar]
- Anti-Smith antibody is associated with disease activity in patients with new-onset systemic lupus erythematosus. Rheumatol Int. 2019;39:1937-44.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of anti-U1-RNP positivity: Systemic lupus erythematosus versus mixed connective tissue disease. Rheumatol Int. 2018;38:1169-78.
- [CrossRef] [PubMed] [Google Scholar]







