Translate this page into:
Rituximab Induced Rare Cystic Lesion in Lungs in a Nephrotic Child: A Case Report
Corresponding author: Subal K. Pradhan, Division of Pediatric Nephrology, SVP Post Graduate Institute of Pediatrics (SVPPGIP) and Sriram Chandra Bhanja (SCB) Medical College, Cuttack, Odisha, India. E-mail: drsubal@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pradhan SK, Nayak S. Rituximab Induced Rare Cystic Lesion in Lungs in a Nephrotic Child: A Case Report. Indian J Nephrol. 2024;34:528-32. doi: 10.25259/IJN_576_20
Abstract
Rituximab has been extensively used for managing B-cell lymphomas due to its anti-CD20 monoclonal antibody activity. Over the last decade, its application has been extended to manage frequent relapsing or steroid-dependent nephrotic syndrome. Its use has been comparatively safe, but few cases of adverse effects on the lung have been reported in the adult population. These lung injury presentations are rarely reported in a pediatric group with only four cases in the literature. Below is a rare case of rituximab-induced lung injury in a 9-year-old boy with frequent relapse of nephrotic syndrome, which developed after four days of rituximab infusion. Suspecting infection and sepsis, several antibiotics were started, but with no improvement in respiratory complaints, even antifungal and antituberculosis treatments were initiated. Finally, setting up a casual relation with the time of infusion to the development of complaints, association with rituximab was suspected. The patient responded to steroid therapy with complete resolution of respiratory complaints. To our knowledge, this is the first reported case of rituximab-induced cystic lesion in lungs from India.
Keywords
Cystic lesion
nephrotic syndrome
rituximab children
ALI
Introduction
Nephrotic syndrome (NS) has an average worldwide incidence of 2–16.9/100,000 children and is more prominent in Asians (incidence of 16 cases/100,000 children/year).1,2 For idiopathic NS (INS), corticosteroids are the treatment of choice, and the majority of them respond well (complete resolution of proteinuria), labeled as steroid-sensitive NS (SSNS).3 But around half of these cases may develop multiple relapses (steroid-dependent NS [SDNS]), having at least two consecutive relapses during tapering or within 14 days of cessation of steroids. Few may have frequent relapses; four relapses/year or two relapses within six months of the initial development of symptoms, termed frequent relapsing NS (FRNS). The standard management for children with FRNS/SDNS includes immunosuppressive like cyclosporine (CyA), tacrolimus, mycophenolate mofetil (MMF), and cyclophosphamide. But around 10–20% of children of FRNS on CyA have frequent relapses, while few cases report a lack of efficacy or side effects on long-term usage.4,5 In cases with resistance or dependence on steroids, it poses the need to discover a novel drug for the treatment of FRNS/SDNS to prevent permanent renal damage.
Rituximab, a chimeric anti-CD20 IgG1 monoclonal antibody, has been effective in the treatment of several autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, and lymphomas.6-8 Rituximab has also shown satisfactory results in pediatric cases of FRNS or SDNS. It is comparatively safe in most adult cases, but in pediatric group, it is associated with several adverse effects such as pulmonary fibrosis,9 pneumocystis pneumonia,10 fulminant myocarditis,11 and ulcerative colitis.12 Post-rituximab pulmonary toxicities are rare and majorly seen in the elderly patient group. Here, we present a pediatric case of FRNS not responding to other immunosuppressive therapy, for which rituximab was infused. The patient developed symptoms of acute lung injury induced by rituximab, which resolved with prednisolone therapy.
Case Report
A 9-year-old male child, a known case of FRNS, was admitted for the first dose of rituximab therapy following remission on a daily dose of prednisolone and tacrolimus. He was diagnosed with NS at the age of 2.5 years and has had multiple episodes of relapse since then. Initially, he was on oral cyclosporine. But due to frequent relapses despite cyclosporine therapy and the development of side effects, he was switched to oral cyclophosphamide followed by tacrolimus. He was in remission for 3 years but again had two episodes of relapse.
His renal biopsy was suggestive of focal segmental glomerulosclerosis (FSGS). But increasing the frequency of relapse (>4) despite receiving two different calcineurin inhibitors, planned to give a single dose (375 mg/m2) of rituximab infusion in the remission phase. The patient was completely asymptomatic and was off from corticosteroids at the time of rituximab injection. His vitals and urine reports were within normal range, and even after injecting rituximab, there were no signs of an acute reaction. Hence, he was discharged. But after three days, he reported fever, cough, and respiratory distress.
On examination, he had tachycardia, tachypnea with intercostal retractions, mild abdominal tenderness, and bilateral coarse crepitations on chest auscultation. The peripheral perfusion was well maintained, and the child was conscious and alert. Thus, a probable diagnosis of NS (frequent relapse) with pneumonia and sepsis was made. Intravenous ceftriaxone was initiated with supportive management, and a stress dose corticosteroid was started. His initial blood investigations suggested pancytopenia with an absolute neutrophil count of 927/cm3. Serum albumin was 3.8 g/dL, and the serum creatinine was 0.8 mg/dL. The liver function test was within normal limits. C-reactive protein was raised (10 mg/dL) with ESR 30 mm/1st hour. Blood, urine, and sputum culture was sent. Urine examination revealed 1+ proteinuria with no pus cells. His chest X-ray [Figure 1a] showed diffuse bilateral hazy opacities with few bullous lesions in the left upper and midzone.

- (a) X-ray chest showing diffuse bilateral hazy opacities with few bullous lesions in left upper and midzone and (b) repeat X-ray showing persistent hazy opacities with few areas of cavitary lesions.
Abdominal tenderness improved, but fever and tachypnea persisted, requiring oxygen. Neutropenia persisted in the repeat blood examination. Hence, antibiotics were upgraded to piperacillin + tazobactam and amikacin.
Since pancytopenia followed after a few days of receiving rituximab. Hence it was suspected to be the probable cause apart from infection-induced bone marrow suppression. But after 7 days of antibiotics, the fever improved, but tachypnea with retractions persisted. A repeat chest X-ray [Figure 1b] revealed persistent hazy opacities with few areas of cavitations. Thus, suspecting tuberculosis, Mantoux, sputum CBNAAT were performed, which were negative. His sputum gram staining, and fungal staining were negative and no growth was seen in culture. High-resolution computed tomography (HRCT) suggested multicyclic lesions with surrounding consolidation and bilateral ground glass appearance [Figures 2 and 3]. He had persistent respiratory distress with bilateral course crepitations, hypoxemia, and pancytopenia after two weeks of broad-spectrum antibiotics.
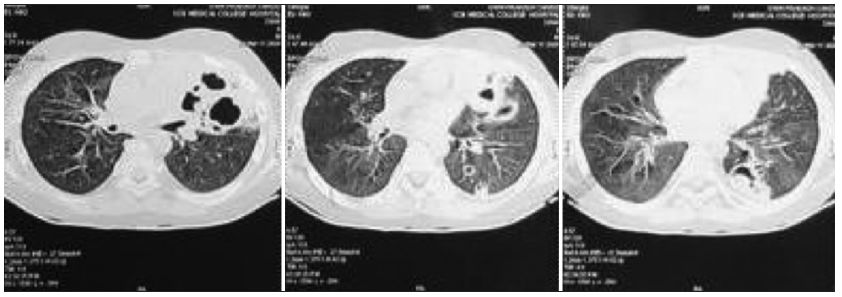
- High-resolution computed tomography (HRCT) of lungs revealing multicystic lesions with surrounding consolidation and air bronchogram.
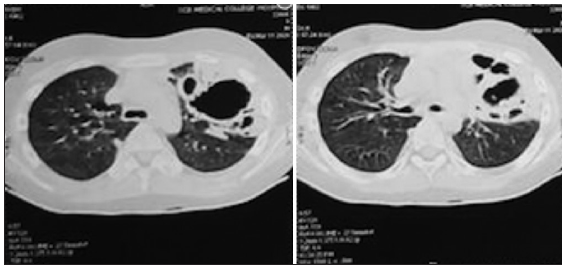
- High resolution computed tomography (HRCT) shows bilateral ground glass appearance.
Hence, a repeat blood and sputum culture, and sputum CBNAAT were performed, which were negative. Anti-tuberculous treatment (ATT) was started empirically with the appropriate weight band as per the revised national TB control program (RNTCP). But the patient showed no improvement after two weeks of treatment. Thus, considering the immunosuppressed state and presence of cystic lesions in the lung, antifungal fluconazole was initiated, but the patient didn’t respond to the therapy. Thus, considering the history of rituximab therapy four days prior to the development of his illness and pancytopenia, a diagnosis of rituximab-induced acute lung injury was made.
All antibiotics, including ATT and antifungal drugs, were discontinued. The patient was administered oral prednisolone (1 mg/kg/day) and gradually decreased his respiratory distress. After seven days of oral prednisolone, the patient was weaned off oxygen and discharged. On follow-up visits, the patient was doing well and even his chest X-ray was normal, though CD19 (total B cells) count was 0% and absolute CD19 was 0.00/μL at 1-month follow-up. At the 3-month follow-up, he was in remission. Currently, he is on oral tacrolimus (0.05 mg/kg) and asymptomatic.
Discussion
Rituximab is a chimeric (human/murine) anti-CD20 monoclonal antibody that acts against the B-lymphocyte cells and is used for managing B-cell lymphomas and other autoimmune diseases. Over the last decade, its application has been extended to the management of FRNS/SDNS in pediatric cases too.10 Rituximab-associated lung injury (RALI) is a rare and serious complication, majorly seen in elderly patients with lymphomas, malignancies or rheumatological diseases with a mortality of 10–20%.13 Its incidence is low due to spontaneous resolution of steroids or its recognized after its discontinuation.14 In a case series reporting five patients, showed severe pulmonary toxicity of RTX-rituximab which developed within weeks of initiating the treatment.15,16 Exact pathogenesis of Rituximab induced lung injury is not known but autopsy data have revealed intra-alveolar hemorrhage with diffuse alveolar damage. There is formation of granulomas in a background of lymphocytic infiltrate.17
Only four cases of RALI in a pediatric group with NS have been reported since its application in this field is new, with limited cases being treated with it. The above-reported case of RALI was seen in a 9-year-old male child with FRNS with a history of treatment with corticosteroids and calcineurin inhibitors (cyclosporine A and tacrolimus). But despite these treatments, patients complained of recurrent relapses showing inadequate response to treatment. Thus, the decision to administer rituximab was made with no immediate adverse effects during the infusion. But after three days he returned with complaints of fever, pain abdomen, and respiratory distress. Clinical features suggested pneumonia with sepsis, and he was started with antibiotics which were upgraded seeing no response. He was even started on ATT and antifungal treatment with no resolution of symptoms. Despite extensive workup and no response to medications, finally setting up a casual relation with the administration of rituximab and development of symptoms within 4–7 days confirmed the diagnosis to be RALI.
All reported cases of RALI presented with fever, dry cough, tachypnea, and hypoxemia. The radiological appearance of RALI included bilateral ground glass opacities, dense parenchymal nodular infiltrates, diffuse interstitial involvement, and diffuse alveolar involvement.7-9 In the above case, though radiological features don’t match with previously described cases, he responded well to steroid therapy indicating immunologically mediated injury to lung tissue secondary to rituximab injection. On follow-up, he also had resolution of the radiological changes with clinical improvement. Hence, we attribute these cystic lesions in the lungs as a form of rituximab-associated lung injury which clinicians should be aware of and should consider as one of their differential diagnosis while managing such cases.
The exact pathogenesis is unclear, but depending on the rapidity of onset of complaints, it can be presumed that: if it develops within few hours of administration, it could be due to cytokine release; if presenting with acute or subacute pneumonia, up to 2 weeks from infusion it could be due to hypersensitivity, and lastly if the development of pneumonia occurs after several months of infusion it is related to drug toxicity. From the above case, we could assume the cause to be hypersensitivity since the development of respiratory complaints took three days to develop and once the diagnosis was confirmed and steroidal treatment was started, and the patient showed good clinical improvement.
Such adverse responses are unpredictable in patients, though in the above case, rituximab was administered for the first time and he developed hypersensitivity but there are reports of patients developing RALI after 2nd or 3rd dose, showing good tolerance during the initial doses.18 Here the probable reason could be the development of anti-rituximab antibodies (ARA) and human anti-chimeric antibodies (HACA). In a study by Ahn et al.,19 two of the cases developed hypersensitivity reactions during infusion of second dose. On monitoring the AHA (Anti rituximab antibody) titers, they were detected after the second dose of rituximab therapy. Data from a review articles have shown that majorly patients present symptoms after four cycles of rituximab and spontaneous resolution occurred after discontinuing the drug. The majority of patients responded to high dose of corticosteroids for a duration of 1–2 months.14,20
RALI cases are managed with antibiotics and steroids, but early diagnosis and treatment could minimize the mortality of almost 25%. In the above case, the patient did not show adequate response with the persistent of respiratory distress despite antibiotics, antifungal, or ATT; but only responded to steroids after 48 hours. In Table 1,9,18,21 four similar cases are reported that favor the above case findings in pediatric patients. Three of the cases responded to treatment, but in a case by Chaumais et al.,9 the patient died due to severe pulmonary fibrosis. But in that report, there were several concomitant factors such as anticoagulant overdose and the presence of respiratory synchytial virus, which precipitated in the fatality.
| Reference | Age (years) | Sex | Rituximab dose | No. of pulse given prior to development of RALI | Duration from last dose | Clinical presentation | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Spatafora et al. (Case 1)18 | 16 | Female | 375 mg/m2 | 3 | 2 weeks | Palpitation, hypotension, fever | Antibiotics, steroids | Complete recovery |
| Spatafora et al. (Case 2)18 | 15 | Male | 375 mg/m2 | 2 | 2 weeks | Fatigue, weakness, dyspnea | No treatment | Complete recovery |
| Bitzan et al.21 | 14 | Male | 375 mg/m2 | 6 | 18 days | Dyspnea, hypoxemia, fever, fatigue | Intravenous immunoglobulins | Complete recovery |
| Chaumais et al.9 | 9 | Female | 375 mg/m2 | 1 | 3 days | Respiratory distress, hypoxia | Antibiotics, antiviral against syncytial virus | Death |
RALI: Rituximab-associated lung injury.
Thus, while managing pediatric cases of NS with Rituximab, lung injury should be considered as its probable adverse effect.
Conclusion
RALI should be considered as an adverse effect of rituximab in treatment of NS. Though its incidence in adults is more, while only four pediatric cases are reported in the literature, early diagnosis is the key to treatment. Differentiating the cause of development of lung injury from infection, immunosuppression, and association with other therapies for NS can be challenging. Prompt diagnosis and immediate treatment with steroids achieve complete recovery and prevent permanent renal damage.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Ethnic differences in childhood nephrotic syndrome. Front Pediatr. 2016;4:39.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- NS in children: Prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int. 1978;13:159-65.
- [CrossRef] [PubMed] [Google Scholar]
- The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr. 1981;98:561-4.
- [CrossRef] [PubMed] [Google Scholar]
- Effective and safe treatment with cyclosporine in nephrotic children: A prospective, randomized multicenter trial. Kidney Int. 2008;73:1167-73.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for cyclosporine-induced tubulointerstitial lesions in children with minimal change nephrotic syndrome. Kidney Int. 2002;61:1801-5.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab: Expanding role in therapy for lymphomas and autoimmune diseases. Annu Rev Med. 2004;55:477-503.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review: Efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146:25-33.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab: A new therapeutic alternative in rheumatoid arthritis. Joint Bone Spine. 2008;75:526-32.
- [CrossRef] [PubMed] [Google Scholar]
- Fatal pulmonary fibrosis after rituximab administration. Pediatr Nephrol. 2009;24:1753-5.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: A multicentric series of 22 cases. Pediatr Nephrol. 2008;23:1269-79.
- [CrossRef] [PubMed] [Google Scholar]
- Fulminant viral myocarditis after rituximab therapy in pediatric nephrotic syndrome. Pediatr Nephrol. 2013;28:1875-9.
- [CrossRef] [PubMed] [Google Scholar]
- Severe ulcerative -colitis after rituximab therapy. Pediatrics. 2010;126:e243-6.
- [CrossRef] [PubMed] [Google Scholar]
- Non-infectious pulmonary toxicity of rituximab: A systematic review. Rheumatology (Oxford). 2012;51:653-62.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab-induced interstitial lung disease. Am J Hematol. 2007;82:916-9.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab-induced interstitial lung disease: Five case reports. Eur Clin Respir J. 2015;2:1-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rituximab-induced lung disease: A systematic literature review. Eur Respir J. 2010;35:681-7.
- [CrossRef] [PubMed] [Google Scholar]
- Fatal intra-alveolar hemorrhage after rituximab in a patient with non-Hodgkin lymphoma. Leuk Lymphoma. 2004;45:2321-5.
- [CrossRef] [PubMed] [Google Scholar]
- A mild form of rituximab-associated lung injury in two adolescents treated for nephrotic syndrome. BMJ Case Rep 2015:bcr2015212694. doi: 10.1136/bcr-2015-212694
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Development of antirituximab antibodies in children with nephrotic syndrome. Pediatr Nephrol. 2014;29:1461-4.
- [CrossRef] [PubMed] [Google Scholar]
- Interstitial pneumonitis and alveolar hemorrhage complicating use of rituximab. Case report and review of the literature. Respiration. 2008;76:449-53.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab (B-cell depleting antibody) associated lung injury (RALI): A pediatric case and systematic review of the literature. Pediatr Pulmonol. 2009;44:922-34.
- [CrossRef] [PubMed] [Google Scholar]







