Translate this page into:
Role of Desmopressin Acetate before Percutaneous Ultrasound-Guided Kidney Biopsy in Patients with Kidney Dysfunction
Corresponding author: Dr. Manish Rathi, Department of Nephrology, Post Graduate Institute of Medical Education and Research, Chandigarh, India. E-mail: drmanishrathi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sethi J, Bansal S, Lal A, Kohli HS, Rathi M. Role of Desmopressin Acetate before Percutaneous Ultrasound-Guided Kidney Biopsy in Patients with Kidney Dysfunction. Indian J Nephrol. 2024;34:228–32. doi: 10.4103/ijn.ijn_34_23
Abstract
Background:
The most common complication of percutaneous kidney biopsy is bleeding, which can be seen in up to one-third of cases. The aim of this study was to evaluate the effect of prebiopsy administration of intranasal desmopressin acetate in reducing the incidence of biopsy-related bleeding complications.
Materials and Methods:
This was a prospective randomized double-blind pilot study conducted at our center from January 2021 to September 2022. Consecutive adult patients who underwent native percutaneous kidney biopsy with an estimated glomerular filtration rate (eGFR) ≤45 ml/min/1.73 m2 were randomized into a placebo (saline intranasal spray) group versus intranasal desmopressin group. The bleeding complications were compared between the two groups.
Results:
A total of 80 patients who underwent kidney biopsy at our center from January 2021 to September 2022 with eGFR ≤45 ml/min/1.73 m2 were included (40 patients in desmopressin group and 40 patients in non-desmopressin group) in the study. The mean age of the patients was 44 ± 12 years with a mean eGFR of 20.82 ± 12.64 ml/min/1.73 m2. Intranasal desmopressin administration before kidney biopsy was associated with a significantly higher number of minor bleeding complications (P = 0.02) and no significant reduction in major complications (P = 0.15) when compared with a group that did not receive desmopressin. Other complications like hypotension, flushing, and vasovagal syncope were not statistically significantly associated with the use of desmopressin.
Conclusion:
Our study did not find any utility of prophylactic desmopressin use before kidney biopsy in patients with kidney dysfunction.
Keywords
Complications
desmopressin
intranasal
kidney biopsy
renal failure

Introduction
Percutaneous native kidney biopsy (NKB) is the gold standard for diagnosis of kidney disease but is associated with bleeding complications such as macroscopic hematuria (3.5%), post-biopsy hematoma (11.6%), erythrocyte transfusion (0.9%), and rarely nephrectomy (0.01%) or death (0.02%).1 Our center’s preliminary data have shown the incidence of post-biopsy complications of around 15% overall, which includes major (2%) and minor (13%) complications. Major complications included mortality and post-biopsy bleeding requiring blood transfusion or intervention in the form of angioembolization/nephrectomy. Minor complications include asymptomatic hematomas, hematuria not associated with a drop in hemoglobin, and vasovagal syncope/pain post-biopsy. Various measures have been employed to reduce the post-biopsy bleeding complications that include desmopressin acetate, recombinant activated factor VII, controlling blood pressure, and avoiding the use of antiplatelet drugs before the procedure.2 Of these, nephrologists often prescribe desmopressin before the NKB. Also known as 1-deamino-8-D-arginine vasopressin (DDAVP), this synthetic derivative of the antidiuretic hormone (ADH) vasopressin improves uremia-related platelet dysfunction by increasing levels of von Willebrand factor and factor VIII.3 Desmopressin may be given intravenously or subcutaneously at a dose of 0.3 µg/kg (in 50 ml of saline over 15–30 min if intravenously). The intranasal dose is 150 µg for patients weighing <50 kg and 300 μg for patients weighing ≥50 kg.4 The peak effect is achieved 30–60 min after intravenous infusion, and 60–90 min after an intranasal or subcutaneous dose. DDAVP nasal spray (desmopressin acetate) is a synthetic analog of the natural pituitary hormone 8-arginine vasopressin (ADH).
Manno et al.5 in a randomized trial suggested a beneficial effect of subcutaneous desmopressin before NKB in low-risk patients with eGFR ≥60 ml/min per 1.73 m2. Later, Peters et al.6 compared bleeding complications between centers that did and did not administer intranasal desmopressin and reported that prophylactic desmopressin administration before NKB reduces overall bleeding in patients with a serum creatinine >150 µmol/l, with a stronger effect in women than in men. Rao et al.7 showed that intranasal desmopressin reduces bleeding complications in patients with a serum creatinine of >132.6 µmol/l but was associated with a significant risk of developing hyponatremia. The above studies did not include patients on hemodialysis and most of them were retrospective in nature. Due to the lack of sufficient evidence and robust data, the guidelines remain silent on the routine use of desmopressin before kidney biopsy. However, they suggest using desmopressin only for patients with Stage 3b chronic kidney disease (CKD) and onwards.8 The present study was done to evaluate the efficacy of prophylactic desmopressin in reducing bleeding complications after percutaneous kidney biopsy in patients with renal dysfunction.
Materials and Methods
This was a prospective randomized double-blind pilot study that was carried out in the Department of Nephrology at PGIMER, Chandigarh from January 2021 to September 2022. Consecutive adult patients who underwent native percutaneous kidney biopsy for any indication and with an estimated glomerular filtration rate (eGFR) ≤45 ml/min/1.73 m2 as per Chronic Kidney Disease Epidemiology were enrolled [Figure 1]. Patients with uncontrolled hypertension, multiple bilateral cysts, horseshoe kidney, hydronephrosis, shrunken kidneys, pregnancy, anemia with hemoglobin <8 g/dl, thrombocytopenia (platelet count <85,000/µl), active urinary tract infection, and abnormal coagulation parameters were excluded. Patients whose kidneys were considered biopsable, i.e., size more than 9 cm bilaterally with good corticomedullary differentiation, were included. All patients with borderline kidney size suggestive of underlying CKD were excluded. Demographic data of the patient, indications for biopsy, laboratory reports, and clinical and hemodynamic parameters were collected from the individual files. All recruited subjects were randomized using computer-generated random allocation numbers into two groups, i.e., the non-desmopressin placebo group versus the desmopressin treatment group. It was a double-blind study as both the patient and the doctor were not aware of the treatment arm the patient was randomized before biopsy. It was a pilot study, hence a sample size of 40 was taken in each group. For blinding, vials containing normal saline and desmopressin were labeled as A and B, respectively. The patients in the placebo group were administered normal saline spray (150 µg) 1 h before biopsy, while in the desmopressin group, desmopressin acetate (150 µg) was administered intranasally 1 h before renal biopsy. Nasal spray is available as 0.01% solution delivering 10 ug per spray and hence 15 nasal sprays were administered to the patient an hour before the biopsy. Careful attention was paid to fluid balance and excessive fluid intake was discouraged for 6–8 h after its administration. The kidney biopsies were performed using a percutaneous technique with real-time ultrasonography (Sonosite Micro Maxx ultrasound machine) imaging with a 3.75 MHz curvilinear probe. All biopsies were performed by a trainee nephrologist who had completed a minimum of 25 biopsy procedures before and was adequately trained. The biopsies were taken using an automated spring-loaded biopsy gun (Bard Peripheral Vascular Inc., USA, BARD®. Max-core™ disposable core biopsy needle) with a 16 gauge and 16 cm long needle with a penetration depth of 22 mm. Firm compression was applied for a minimum of 30 min over the puncture site with a sterile gauge. The patient was then made to lie in a supine position and vital monitoring was done, viz., every 15 min for the first 1 h, every 30 min for the next 2 h, and hourly thereafter for the next 5 h. The patient was kept on bed rest for 8 h with pulse and blood pressure monitoring and the urine was tested for the presence of gross hematuria. A post-biopsy screening ultrasound abdomen to assess for hematoma was performed in all the patients. Repeat ultrasound or computed tomography was done if any signs or symptoms of a bleeding complication such as a significant decrease in hemoglobin, hypotension, flank pain, or hematuria were present. Outpatients were discharged home 8 h after the procedure provided; there were no signs of bleeding or flank pain with the advice to take bed rest for the first 24 h and avoid any strenuous activity for one week. The primary outcome of the study was to assess the incidence of post-biopsy major bleeding complications as defined by a significant decline in hemoglobin level requiring blood transfusions, arteriovenous fistula, and the need for radiological intervention like gel foam or coil embolization or nephrectomy. Secondary outcomes were the incidence of minor post-biopsy bleeding complications as defined by hematuria and post-biopsy ultrasound screening hematoma. The study was approved by the Institute’s Ethics Committee. Unpaired Student’s t-test was used for comparative analysis between the two groups. Pearson’s Chi-square test or Fisher exact test was employed to analyze categorical data as appropriate. A P value <0.05 was considered significant. Statistical Package for the Social Sciences, Version 22 (SPSS 22, IBM Chicago, USA) was used for statistical analyses.
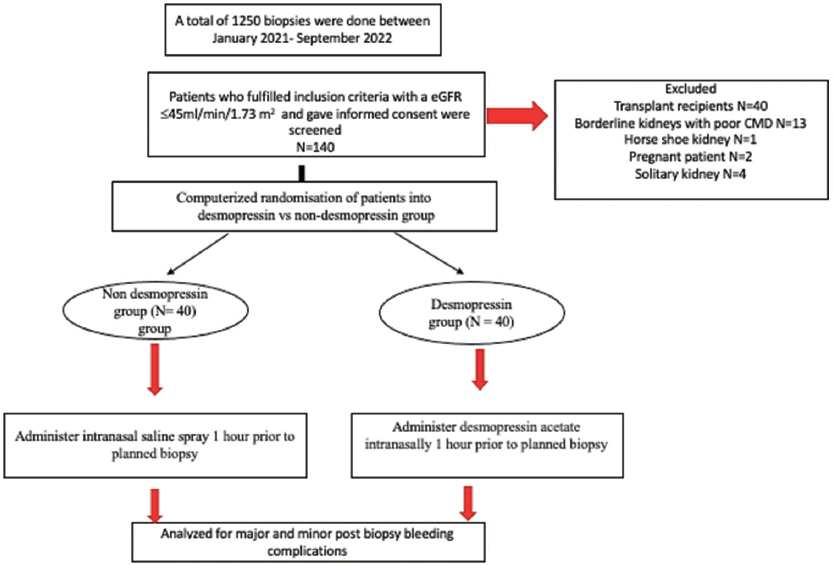
- Consort diagram of the study cohort. eGFR: estimated glomerular filtration rate. CMD: cortico-medullary differentiation.
Results
A total of 80 patients who underwent kidney biopsy at our center from January 2021 to September 2022 with eGFR ≤45 ml/min/1.73 m2 were included (40 patients in the desmopressin group and 40 patients in the placebo group). The baseline characteristics and demographics of the study cohort in both groups are compared in Table 1. The mean age of the patients was 44 ± 12 years with almost equal representation of both sexes. Baseline hemoglobin was low in the placebo group (9.55 ± 1.40 g/dl) than in the desmopressin group (10.69 ± 2.04 g/dl). Patients with CKD Stage 3b to 5 were equally represented with 36% patients on hemodialysis. The mean eGFR of the study sample at the time of biopsy was 20.82 ± 12.64 ml/min/1.73 m2. There was a non-significant difference in the eGFR, body mass index, platelet count, bleeding time, prothrombin time, activated partial thromboplastin time, and serum creatinine between both groups. Around 56% of the biopsy procedures were done on an inpatient basis. Interestingly, a significantly more number of patients were biopsied as inpatients in the placebo group (67.5%), as compared to the desmopressin group where only 45% of the patients were admitted before the procedure (P value = 0.04). Baseline comorbidity including diabetes and hypertension were equally distributed between the two groups. The placebo group had a higher requirement of hemodialysis before the biopsy (47.5%) as compared to the desmopressin group (25%). Indications for renal biopsy were rapidly progressive renal failure in 37 (46.2%) patients, nephrotic syndrome with acute kidney injury in 25 (31.2%) patients, and unexplained renal dysfunction in 18 (22.5%) patients. Two biopsy passes were taken in 54 patients (67.5%) of the entire cohort followed by three passes in 19 patients (23.7%) and were equally distributed between both the groups.
| Variables (Mean±SD) |
Group | P | ||||
|---|---|---|---|---|---|---|
| Desmopressin n=40 | Placebo n=40 | Entire cohort n=80 | ||||
| Age (years) | 42.90±14.35 | 45.25±11.00 | 44.07±12.76 | 0.277 | ||
| Males (n %) | 26 (65%) | 24 (60%) | 50 (62.5%) | 0.664 | ||
| eGFR (ml/min/1.73 m2) | 22.41±12.37 | 19.23±12.86 | 20.82±12.64 | 0.216 | ||
| Hemoglobin (g/dl) | 10.69±2.04 | 9.55±1.40 | 10.12±1.84 | 0.007 | ||
| Bleeding time (min) | 2.62±0.39 | 2.53±0.50 | 2.57±0.45 | 0.424 | ||
| Platelet count (lakhs/µl) | 2.47±0.97 | 2.47±1.13 | 2.47±1.04 | 0.747 | ||
| Prothrombin time (seconds) | 14.30±2.91 | 14.45±1.52 | 14.37±2.31 | 0.449 | ||
| aPTT (seconds) | 32.28±11.39 | 30.23±7.44 | 31.25±9.62 | 0.163 | ||
| Serum creatinine (mg/dl) | 4.19±4.36 | 4.65±2.83 | 4.42±3.67 | 0.266 | ||
| Duration of symptoms (days) | 89.95±247.41 | 48.6±48.60 | 67.93±178.54 | 0.273 | ||
| Inpatients (n) | 18 (45%) | 27 (67.5%) | 45 (56.3%) | 0.043 | ||
| Baseline hypertension | 29 (72.5%) | 28 (70%) | 57 (71.3%) | 0.805 | ||
| Diabetes | 15 (37.5%) | 8 (20%) | 23 (28.7%) | 0.084 | ||
| Number of passes >2 | 7 (17.5%) | 12 (30%) | 19 (23.7%) | 0.625 | ||
| HD dependent | 10 (25%) | 19 (47.5%) | 29 (36.3%) | 0.03 | ||
| IFTA scoring | ||||||
| Mild (<25%) | 28 (70%) | 31 (77.5%) | 59 (73.75%) | 0.236 | ||
| Moderate (25–50%) | 7 (17.5%) | 8 (20%) | 15 (18.75%) | |||
| Severe (>50%) | 5 (12.5%) | 1 (2.5%) | 6 (7.5%) | |||
eGFR: Estimated glomerular filtration rate, aPTT: Activated partial thromboplastin time, IFTA: Interstitial fibrosis and tubular atrophy, HD: hemodialysis.
Major complications
Out of the 80 patients, three (0.03%) had a major post-biopsy bleeding complication requiring blood transfusion (two patients) or intervention in the form of coiling (one patient). All three complications occurred in the desmopressin group [Table 2]. Among the minor complications, 8 out of 80 patients had hematuria (10%) and 18 patients (22.5%) had hematoma on immediate post-biopsy ultrasound screening not requiring blood transfusion/intervention. None of the patients expired or needed nephrectomy in our study. The group given intranasal desmopressin had a significantly higher number of all bleeding complications (both major and minor) than the placebo group even if we exclude immediate post-biopsy asymptomatic hematomas (Major: 3 of 40, 7.5% vs. 0 of 40, 0% P = 0.15. Minor: 7% of 40, 17.5% vs. 1% of 40, 2.5% P = 0.02). Other complications like hypotension, flushing, and vasovagal syncope were not statistically significantly associated with the use of desmopressin. There was no significant difference in the number of passes taken and the degree of chronicity (IFTA: interstitial fibrosis and tubular atrophy) between the desmopressin and placebo groups.
| Complications | Group | P | |||
|---|---|---|---|---|---|
| Desmopressin group | Placebo group | Entire cohort | |||
| Major | Requirement of blood transfusion Intervention Nephrectomy/ mortality |
2 (5%) | 0 | 2 (2.5%) | 0.152 |
| 1 (2.5%) 0 |
0 0 |
1 (1.25%) 0 |
0.314 - |
||
| Minor | Hematuria Hematoma on immediate post-biopsy ultrasound scan |
7 (17.5%) 16 (40%) |
1 (2.5%) 2 (5%) |
8 (10%) 18 (22.5%) |
0.02 0.04 |
Discussion
In the present single-center study, administration of desmopressin to patients with renal dysfunction (eGFR ≤45 ml/min/1.73 m2) was associated with an increased rate of post-biopsy minor bleeding complications. The increase in bleeding complications with desmopressin use in our study is not consistent with the published literature. Hemoglobin fall was also more in the desmopressin group, however, that can be attributed to the fact that desmopressin is known to cause a hemoglobin fall >1.0 g/dl due to hemodilution which is attributed to its antidiuretic effect.9
Recently, Jose et al.10 published a retrospective study of patients with eGFR <30 ml/min/1.73 m2, who underwent percutaneous kidney biopsy and showed that prebiopsy intranasal administration of desmopressin was associated with a significantly lower overall rate of bleeding complications (12.37% vs. 20.80%; P = 0.025).9 More than half of the patients in either group received hemodialysis before the biopsy in this study similar to ours. However, the majority of the other studies have not shown any significant benefit of using desmopressin before kidney biopsy. Rao et al.7 (mainly retrospective) used intranasal desmopressin in patients with creatinine >1.5 mg/dl (excluding patients on dialysis), and showed a reduced risk of minor but not major complications and a near-universal incidence of hyponatremia in the desmopressin group. Leclerc et al.9 was a retrospective study and found similar rates of symptomatic hematomas and intervention in patients given intranasal desmopressin as those who did not receive desmopressin. Lastly, Cheong et al.11 (retrospective) did not report any differences in the incidence of renal artery embolization, blood transfusion, and total major bleeding events (P = 0.442) between the two groups; however, the blood transfusions occurred more frequently in the desmopressin group. They postulated that the patients in the desmopressin group were significantly older, had higher blood pressure, higher serum creatinine, lower hemoglobin levels, and lower platelet counts than those in the non-desmopressin group which could have contributed to the high bleeding complications in the desmopressin group. The major limitation of all the previous studies was the retrospective and observational nature of the studies, which may have inherent selection bias. In addition, there was no protocol for administering desmopressin, and therefore, desmopressin was administered to people at a high risk of bleeding, as judged by the attending physician. Hence, a prospective randomized study design in high-risk patients was the need of the hour.
This increased bleeding risk in our study of high-risk patients was mainly driven by minor complications, i.e., increased chances of hematuria and hematoma in the desmopressin group. However, a significantly higher number of patients in the placebo group were biopsied as inpatients and it could be postulated that better monitoring (more hours of immobilization and close monitoring) as inpatients led to a lower bleeding risk in this group. Also, significantly more number of patients received hemodialysis before biopsy in the placebo group. There are many factors that contribute to platelet dysfunction in advanced renal dysfunction that include anemia, dysfunctional von Willebrand factor, platelet membrane abnormalities, and the effect of uremic toxins that inhibit platelet aggregation.12 There is some evidence in the past that hemodialysis might correct the uremic bleeding milieu. It is possible that the pre-biopsy hemodialysis led to a lower bleeding risk in the placebo group. Other risk factors like pre-biopsy hemoglobin, the degree of interstitial fibrosis and tubular atrophy, the number of biopsy passes, underlying comorbidities, and serum creatinine had no significant effect on the biopsy complications. The present study showed no benefit and in fact, a paradoxical increased minor bleeding risk associated with desmopressin use. The use of desmopressin was not associated with any adverse events in our study.
The main strength of our study was the prospective double-blind randomized study design and elimination of potential confounding variables like patients with coagulopathy, low platelet count, solitary kidney, uncontrolled hypertension, and transplant recipients. Our cohort is probably the first in the literature to randomize in a prospective manner and analyze the association between desmopressin use and post-NKB bleeding in patients with eGFR ≤45 ml/min/1.73 m2 (including those on dialysis).
The main drawbacks of our study include its single-center design, small sample size, and residual confounding of the study design (unequal division of hemodialysis and admitted patients in the two groups). In patients who require hemodialysis, it is difficult to separately assess the effects of hemodialysis and desmopressin in the prevention of bleeding complications. Moreover, a platelet function analyzer was not used to measure the bleeding risk.
The Caring for Australians with Renal Impairment (CARI) guidelines (2018) recommended that as there is a lack of evidence to support the benefit or harm of desmopressin administration before renal biopsy, care units should continue their existing practice until a higher level of evidence is available.8 In 2011, Whittier suggested that although desmopressin may play a role in patients at a high risk of bleeding (which deserves a study in itself), administering desmopressin off-label to all patients undergoing percutaneous kidney biopsy is premature and possibly hazardous.13 In this study as well, we observed an increased rate of post-biopsy overall bleeding complications in the desmopressin group than that observed in the placebo group. Our study did not find any utility of prophylactic desmopressin use before kidney biopsy in patients with renal dysfunction. A study with a large sample size done exclusively on inpatients requiring hemodialysis can be planned in the future for the generalizability of our results.
Conflicts of interest
There are no conflicts of interest.
References
- Bleeding complications of native kidney biopsy: A systematic review and meta-analysis. Am J Kidney Dis. 2012;60:62-73.
- [CrossRef] [PubMed] [Google Scholar]
- Factors that can minimize bleeding complications after renal biopsy. Int Urol Nephrol. 2014;46:1969-75.
- [CrossRef] [PubMed] [Google Scholar]
- Deamino-8-D-arginine vasopressin shortens the bleeding time in uremia. N Engl J Med. 1983;308:8-12.
- [CrossRef] [PubMed] [Google Scholar]
- How do you treat bleeding disorders with desmopressin? Postgrad Med J. 2007;83:159-63.
- [CrossRef] [PubMed] [Google Scholar]
- Desmopressin acetate in percutaneous ultrasound-guided kidney biopsy: A randomized controlled trial. Am J Kidney Dis. 2011;57:850-5.
- [CrossRef] [PubMed] [Google Scholar]
- Desmopressin (Octostim®) before a native kidney biopsy can reduce the risk for biopsy complications in patients with impaired renal function: A pilot study. Nephrology (Carlton). 2018;23:366-70.
- [CrossRef] [PubMed] [Google Scholar]
- Intranasal desmopressin reduces renal biopsy-related bleeding and serum sodium levels in patients with reduced renal function. Clin Kidney J. 2019;13:1063-7.
- [CrossRef] [PubMed] [Google Scholar]
- KHA-CARI guideline recommendations for renal biopsy. Nephrology (Carlton). 2019;24:1205-13.
- [CrossRef] [PubMed] [Google Scholar]
- Use of desmopressin prior to kidney biopsy in patients with high bleeding risk. Kidney Int Rep. 2020;5:1180-7.
- [CrossRef] [PubMed] [Google Scholar]
- Desmopressin acetate before percutaneous ultrasound-guided kidney biopsy in patients with renal failure-Is it really beneficial? Indian J Nephrol. 2022;32:430-4.
- [CrossRef] [PubMed] [Google Scholar]
- No effect of desmopressin administration before kidney biopsy on the risk of major post-biopsy bleeding. Nefrologia (Engl Ed). 2022;42:33-40.
- [CrossRef] [PubMed] [Google Scholar]
- Renal biopsy practice: What is the gold standard? World J Nephrol. 2014;3:287-94.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous kidney biopsy: “The needle and the damage done”? Am J Kidney Dis. 2011;57:808-10.
- [CrossRef] [PubMed] [Google Scholar]







