Translate this page into:
Role of Induction in a Haplomatch, Related, Low-Risk, Living-Donor Kidney Transplantation with Triple Drug Immunosuppression: A Single-Center Study
Corresponding author: Dr. Pranaw K. Jha, Senior Consultant & Head, Department of Nephrology, Arihant Multispeciality Hospital, Medical Square, Nagpur - 440 026, Maharashtra, India. E-mail: dr.pranaw@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jha PK, Bansal SB, Sharma R, Sethi SK, Bansal D, Nandwani A, et al. Role of Induction in a Haplomatch, Related, Low-Risk, Living-Donor Kidney Transplantation with Triple Drug Immunosuppression: A Single-Center Study. Indian J Nephrol. 2024;34:246–51. doi: 10.4103/ijn.ijn_84_23
Abstract
Background:
The role of induction in low-risk, living-donor kidney transplants being treated with tacrolimus, mycophenolate mofetil, and prednisolone is debatable.
Materials and Methods:
This was a retrospective study that consisted of patients undergoing living kidney transplantation between February 2010 and June 2021 with a related haplomatch donor, with maintenance immunosuppression of tacrolimus, mycophenolate mofetil, and prednisolone. High-risk transplants, such as second or more transplants, immunologically incompatible transplants, and steroid-free transplants, were excluded. Patients were divided into three groups: no induction, basiliximab induction, and thymoglobulin induction, and the outcomes of all three were compared.
Results:
A total of 350 transplants were performed. There was a significant difference in the recipient sex distribution (P = 0.0373) and the number of preemptive transplants (P = 0.0272) between the groups. Other parameters were comparable. Biopsy-proven acute rejection (BPAR) was significantly less frequent in the thymoglobulin group than in the no-induction (5.3% vs. 17.5%; P = 0.0051) or basiliximab (5.3% vs. 18.8%; P = 0.0054) group. This persisted even after we performed multivariate regression analysis (thymoglobulin vs. no-induction group, P = 0.0146; thymoglobulin vs. basiliximab group, P = 0.0237). There was no difference in BPAR between the basiliximab and no-induction groups. There were no differences in other outcomes between the groups.
Conclusion:
In a low-risk haplomatch, related, living-donor kidney transplant on tacrolimus, mycophenolate mofetil, and prednisolone, BPAR was significantly lower with thymoglobulin as opposed to no induction or basiliximab induction with a similar short-term patient and death-censored graft survival and infection rates. Basiliximab did not provide any benefit over no induction.
Keywords
ATG
basiliximab
haplomatch
induction
kidney transplant
Introduction
Kidney transplantation is the best kidney replacement therapy for patients with stage 5 chronic kidney disease. Induction therapy is an important component of transplant immunosuppression that reduces biopsy-proven acute rejection (BPAR), which in turn affects long-term graft survival.1
The role of induction in low-risk, living-donor kidney transplant recipients in the present era of tacrolimus, mycophenolate mofetil (MMF), and prednisolone-based immunosuppression is not clear.2 The KDIGO guidelines recommend including induction with biological agents as a part of initial immunosuppression. It also recommends using interleukin-2 receptor antagonist (IL2RA) as the first-line agent and using lymphocyte-depleting agent for high-immunological-risk patients.3 The studies on which these recommendations are based on are mostly from the cyclosporin era.4 Also, higher doses of anti-thymocyte globulin (ATG) used in these studies could have been a factor for higher rates of CMV and other infections when compared to IL2RA or no induction. Low-dose (3 mg/kg) rabbit ATG has been found to have acceptable acute rejection rates in a low-risk, living-donor kidney transplant setting.5
The present study was conducted to compare the outcomes between low-risk kidney transplant recipients receiving grafts from a haplomatch, related donor receiving either no induction or basiliximab or low-dose thymoglobulin (Sano-Aventis, Boston, USA), and on a standard triple drug immunosuppression of tacrolimus, MMF, and prednisolone.
Materials and Methods
This was a single-center retrospective study. The kidney transplant program was initiated at our center in February 2010. The present study included patients aged older than 18 years and who underwent a living-related kidney transplant until June 2021 with at least 6 months of follow-up. Patients had received transplant kidney from related (parents, siblings, children, others) haplomatch donors. All high-risk patients were excluded from this study: these included patients with a positive flow cytometry or complement-dependent cytotoxicity (CDC) crossmatch, second or more transplants, ABO-incompatible transplants, human leukocyte antigen (HLA)–incompatible transplants, and steroid-free transplants. Only patients initiated on maintenance immunosuppression with tacrolimus, mycophenolate mofetil/sodium (MMF/MMF-S), and prednisolone were included. Patients who received induction therapy other than basiliximab or thymoglobulin were excluded. Fourteen patients who had received Grafalon (Neovii Pharmaceuticals AG, Switzerland, formerly known as ATGFresenius or ATG-F) induction were excluded.
After complete donor and recipient evaluation, immunological tests were conducted. Human leukocyte antigen A, B, and DRB1 typing were conducted for all donor recipient pairs. For the current study, only those patients who had undergone both crossmatches (CDC and flow cytometry) and were found to be negative were included.
Immunosuppression protocols
Patients received the first dose of thymoglobulin or basiliximab on the day of transplantation. The total dose of thymoglobulin administered was 3 mg/kg IV (1.5 mg/kg each on the day of transplant and postoperative day [POD] 2, whereas basiliximab was administered at 20 mg on the day of transplant and on POD 4). All patients also received 500 mg of IV methylprednisolone intraoperatively, followed by 100 mg of IV hydrocortisone eight-hourly on the day of the transplant surgery. Oral prednisolone was started at a dose of 40 mg daily from POD 1 and tapered to 20 mg daily on the day of discharge. Other maintenance immunosuppressants consisted of tacrolimus and MMF/MMF-S, which was started one day prior to the transplant. Tacrolimus was started at a dose of 0.1 mg/kg in two divided doses, while MMF/MMF-S was started at 1 gm/720 mg, twice daily.
Follow-up
Patients were followed up twice weekly for the first month, once weekly for the second month, once in a fortnight for the third month, and once monthly thereafter for a year. After one year, the patients were followed up once every 2–3 months. During each visit, kidney function tests and hemograms were performed. The tacrolimus target trough level was 8–12 ng/ml during the first 3 months, 5–8 ng/ml from 3 to 6 months, and 4–6 ng/ml thereafter. Prednisolone was tapered to 5 mg at the end of the 3rd month. All patients received trimethoprim sulfamethoxazole prophylaxis for 6 months. Valganciclovir was administered as CMV prophylaxis for 3 months for patients receiving thymoglobulin induction and 6 months if the CMV status of the donor and recipient was D+R−.
Statistical analysis for this study was performed using SAS software (SAS Institute Inc., Cary, NC, USA). Data are reported as mean ± standard deviation. Continuous variables were compared using the unpaired t-test, ANOVA, and Mann–Whitney U test while categorical values were compared using the Chi-squared test or Fisher’s exact test. Survival analysis was performed using the Kaplan–Meier method, and groups were compared using the logrank test. P <0.05 was considered as statistically significant. Multivariate regression analysis was performed to detect independent predictors of significant outcomes.
The study was completed in accordance with the 1964 Helsinki declaration and its later amendments. Ethics committee approval of the institution was taken.
Results
A total of 350 patients met the inclusion and exclusion criteria. They were divided into three groups based on the induction used: no induction (n = 143), basiliximab induction (n = 112), and thymoglobulin induction (n = 95). Outcomes were compared separately between the three pairs: no induction versus thymoglobulin, basiliximab versus thymoglobulin, and no induction versus basiliximab. Thirty-one patients (8.9%) were lost to follow-up.
Table 1 shows the patients’ demographic characteristics. There was a significant difference in the mean follow-up duration, donor sex distribution, and number of preemptive transplants between the three groups. The other parameters were comparable.
| No induction (n=143) | Basiliximab (n=112) | Thymoglobulin (n=95) | P | |
|---|---|---|---|---|
| Follow-up (months) | 48.6±29.9 | 67.9±27.9 | 36.3±22.6 | <0.0001* |
| Recipient age (years) | 33.1±10.4 | 35.7±11.9 | 33.8±10.1 | 0.1773 |
| Recipient gender | 123 M; 20 F | 82 M; 30 F | 78 M; 17 F | 0.0373* |
| BMI | 22.3±4.3 | 22.9±3.6 | 23.1±3.9 | 0.3360 |
| Donor age (years) | 50.1±11.5 | 51.5±10.5 | 51.2±11.9 | 0.5931 |
| Donor gender | 39 M; 104 F | 42 M; 70 F | 36 M; 59 F | 0.1307 |
| Preemptive | 12 (8.4%) | 21 (18.8%) | 17 (17.9%) | 0.0272* |
| Duration on dialysis (months) | 4.2±5.1 | 4.5±7.2 | 5.8±8.7 | 0.2797 |
| Diabetic | 18 (12.6%) | 22 (19.6%) | 12 (12.6%) | 0.2455 |
| Donor relation | 0.1188 | |||
| Parents | 104 (72.7%) | 75 (67%) | 65 (68.4%) | |
| Siblings | 32 (22.4%) | 22 (19.6%) | 18 (19%) | |
| Children | 3 (2.1%) | 10 (8.9%) | 4 (4.2%) | |
| Others | 4 (2.8%) | 5 (4.5%) | 8 (8.4%) |
BMI=Body mass index; *Significant
No induction versus thymoglobulin
Table 2 shows the outcome comparison between the no-induction and thymoglobulin induction groups. In univariate analysis, patients receiving thymoglobulin had significantly lower BPARs. Other outcome parameters, including patient and death-censored graft survival, infections, and CMV infection, were comparable between the two groups. On performing a multivariate logistic regression analysis, taking various factors into account (viz. induction type, recipient and donor age and gender, recipient body mass index, dialysis duration, follow-up duration, and preemptive transplants), the type of induction (P = 0.01) and donor age (P = 0.03) were found to be the parameters that were associated with significant differences in BPAR [Supplementary Table 1].
| No induction (n=143) | Thymoglobulin (n=95) | P | |
|---|---|---|---|
| Death-censored graft survival | 134 (93.7%) | 92 (96.8%) | 0.3713 |
| Patient survival | 136 (95.1%) | 89 (93.7%) | 0.7724 |
| BPAR | 25 (17.5%) | 5 (5.3%) | 0.0051* |
| Serum creatinine at 6 months (mg/dl) | 1.38±0.43 | 1.4±0.39 | 0.6650 |
| Serum creatinine at last follow-up (mg/dl) | 1.45±0.65 | 1.55±0.85 | 0.3367 |
| Infections | 62 (43.4%) | 40 (42.1%) | 0.8940 |
| CMV infection | 15 (10.5%) | 6 (6.3%) | 0.3524 |
| NODAT | 21 (14.7%) | 10 (10.5%) | 0.4331 |
BPAR=Biopsy-proven acute rejection; CMV=Cytomegalovirus; NODAT=New-onset diabetes after transplant; *p < 0.05.
Basiliximab versus thymoglobulin
Table 3 shows the outcome comparisons between groups. In univariate analysis, there was a significant difference in BPAR between the two groups (18.8% vs. 5.3%; P = 0.0054), whereas other parameters were comparable. The induction type was still a significant factor in the multivariate logistic regression analysis using various factors as mentioned above (P = 0.02) [Supplementary Table 2].
| Basiliximab (n=112) | Thymoglobulin (n=95) | P | |
|---|---|---|---|
| Death-censored graft survival | 102 (91.1%) | 92 (96.8%) | 0.1484 |
| Patient survival | 105 (93.7%) | 89 (93.7%) | 1.0000 |
| BPAR | 21 (18.8%) | 5 (5.3%) | 0.0054* |
| Serum creatinine at 6 months (mg/dl) | 1.35±0.44 | 1.4±0.39 | 0.4818 |
| Serum creatinine at last follow-up (mg/dl) | 1.41±0.54 | 1.52±0.8 | 0.2269 |
| Infections | 53 (47.3%) | 40 (42.1%) | 0.4852 |
| CMV infection | 14 (12.5%) | 6 (6.3%) | 0.1604 |
| NODAT | 14 (12.5%) | 10 (10.5%) | 0.8280 |
BPAR=Biopsy-proven acute rejection; CMV=Cytomegalovirus; NODAT=New-onset diabetes after transplant; *p < 0.05
No induction versus basiliximab
Table 4 shows comparison of the outcome parameters between patients receiving no induction and those receiving basiliximab induction. There was no significant difference between the two groups.
| No-induction (n=143) | Basiliximab (n=112) | P | |
|---|---|---|---|
| Death-censored graft survival | 134 (93.7%) | 102 (91.1%) | 0.4265 |
| Patient survival | 136 (95.1%) | 105 (93.7%) | 0.6374 |
| BPAR | 25 (17.5%) | 21 (18.8%) | 0.8700 |
| Serum creatinine at 6 months (mg/dl) | 1.37±0.43 | 1.36±0.43 | 0.7598 |
| Serum creatinine at last follow-up (mg/dl) | 1.45±0.65 | 1.41±0.54 | 0.5301 |
| Infections | 62 (43.4%) | 53 (47.3%) | 0.6121 |
| CMV infection | 15 (10.5%) | 14 (12.5%) | 0.6924 |
| NODAT | 21 (14.7%) | 14 (12.5%) | 0.7148 |
BPAR=Biopsy-proven acute rejection; CMV=Cytomegalovirus; NODAT=New-onset diabetes after transplant
Supplementary Table 3 shows a comparison of rejection types between the groups. All rejections (n = 5) in the thymoglobulin group were borderline cellular in nature. All these were indication biopsies, and all the borderline rejection cases were treated with anti-rejection therapy.
Figures 1-3 show the Kaplan–Meier survival curves for patient survival, death-censored graft survival, and BPAR comparing the three groups. A significant difference was observed for BPAR between the groups (logrank P = 0.0148).
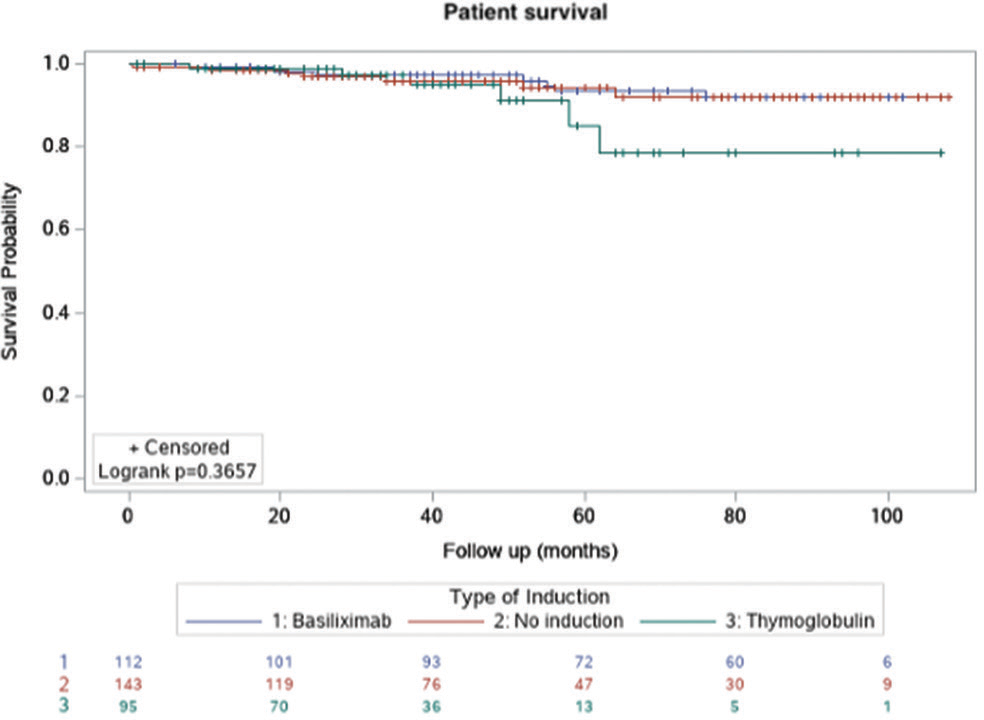
- Kaplan–Meier survival curve for patient survival.
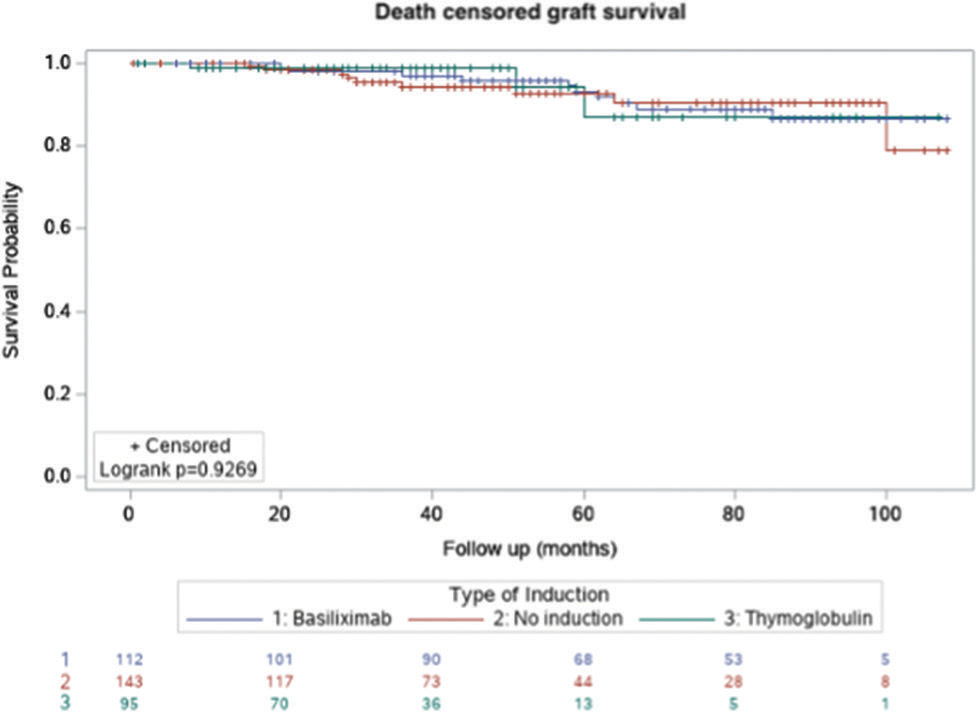
- Kaplan–Meier survival curve for death censored graft survival.
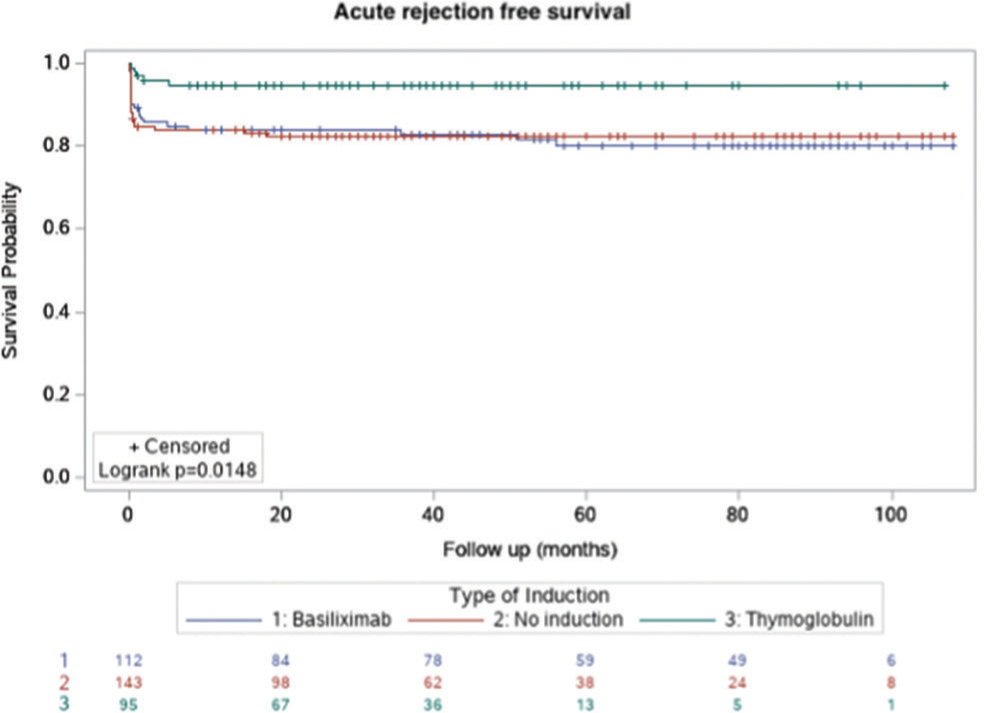
- Kaplan–Meier survival curve for biopsy-proven, acute rejection–free survival.
Discussion
Rabbit ATG is the preferred induction agent in high-risk renal transplant recipients.6,7 However, the role of induction and the preferred induction agent in kidney transplant recipients receiving grafts from related living donors in the present era of immunosuppression is not clear. Our study compared the outcome of basiliximab versus low-dose thymoglobulin versus no induction in a low-risk setting of related, living-donor, haplomatch kidney transplant with standard triple drug immunosuppression consisting of tacrolimus, MMF, and prednisolone.
There was a significant difference in the follow-up duration between the groups. The thymoglobulin group had the shortest period of follow-up whereas the basiliximab group had the longest. There was a significant difference in the recipient sex distribution and the number of preemptive transplants between the groups. The other baseline parameters were comparable between groups.
There was no significant difference in patient survival or death-censored graft survival between the groups. This could be due to the relatively shorter follow-up duration. In a study conducted by Koyawala et al.,8 which included 35% living donor transplants, the risk of death and allograft failure was lower with thymoglobulin induction than with basiliximab or alemtuzumab induction. Another meta-analysis revealed no significant difference in patient and graft survival when using IL2RA versus rATG with tacrolimus-based maintenance immunosuppression.6 Studies comparing patients receiving basiliximab and those receiving no induction with triple drug maintenance immunosuppression have also shown no difference in patient and graft survival.9,10 A randomized control trial is currently being conducted to compare the outcomes between patients receiving basiliximab and those receiving no induction in a low-immunological-risk kidney transplant setting.11
In the present study, the BPAR rate was significantly lower with thymoglobulin induction than with no induction or basiliximab induction. This difference persisted even after multivariate regression analysis was adjusted for various baseline parameters, including the follow-up duration and number of preemptive transplants. All rejections in the thymoglobulin group were borderline cellular in nature. Various studies have highlighted the superiority of rATG over IL2RA in the prevention of acute rejection in kidney transplant recipients.12,13
There was no significant difference in the BPAR rate between the no-induction and basiliximab groups in our study. Other studies have also shown that basiliximab has no advantage over no induction in the present era of tacrolimus, MMF, and prednisolone immunosuppression.14,15
Tanriover et al.16 carried out a registry analysis on the role of induction therapies in living-donor kidney transplantation who were on tacrolimus- and mycophenolate-based immunosuppression with or without steroid. Authors concluded that IL2RA induction was not associated with better outcomes compared to no induction therapy, and rabbit ATG was proposed to be a preferred induction alternative in steroid avoidance protocols. The findings of the study were in line with our study, though the former study did not provide the dose of thymoglobulin that was used and the rate of infections in various groups, unlike our study. Evans et al.17 looked at outcomes of low-risk transplant recipients matched with their donor for A, B, DR, and DQB1 antigens using US Organ Procurement and Transplantation Network data. Authors found that in this subset of patients, the outcomes were similar between all the groups, irrespective of induction. This has been the traditional thinking, that such well-matched patients do not need induction.
On performing a multivariate regression analysis assessing BPAR in the thymoglobulin and no-induction group, donor age was also found to be a significant factor. Older donor age has been reported to be associated with higher acute rejection rates in other studies as well.18,19
In the present study, there was no significant difference in the infection rate between the groups. In addition to this, there was no significant difference in the rate of CMV infection, although the thymoglobulin group had fewer CMV infections. This could be because of the universal prophylaxis administered to patients receiving thymoglobulin and the low dose of thymoglobulin used in the present study. Other studies have reported a higher rate of CMV and other infections in patients receiving rATG.13,20,21
Our study has a few limitations. First, this was a retrospective study and was, hence, prone to bias. We performed a multivariable analysis to reduce bias by considering various baseline factors that could affect the outcomes. Second, the duration of follow-up was relatively short to assess long-term outcomes, such as patient and graft survival. It would be interesting to determine whether the higher BPAR in the no-induction and basiliximab groups compared to the thymoglobulin group also translates into poor graft and patient survival on long-term follow-up. Third, the outcome of the present study applies to a selective population consisting only of the low-risk group.
Despite the above-mentioned limitations, the present study is important because it explored the role of induction in a subset of patients, which has been traditionally deemed to be a low-risk group. The thymoglobulin dose was lower than that reported in the previous studies. Our study showed that the risk of BPAR was minimal with thymoglobulin induction, though with equal graft and patient survival and infection rates when compared to basiliximab or no induction. There was no advantage of basiliximab over no induction in the prevention of BPAR or other outcomes. Randomized studies with a larger number of patients and longer follow-up periods are needed.
Conflicts of interest
VK has received research funding from Novartis India, Sanofi Aventis India, Astellas India. He has been scientific advisor for Roche India, Novartis India, Astellas India, Torrent India, Reddy’s India, Biocon India, Medtronics, Wockhardt India and declared having received honoraria and speaker fees from them. AK has been in speaker forum for Sanofi. All the other authors declared no competing interests pertaining to the publication.
References
- Long-term outcomes after acute rejection in kidney transplant recipients: An ANZDATA analysis. J Am Soc Nephrol. 2019;30:1697-707.
- [CrossRef] [PubMed] [Google Scholar]
- Utility or futility of interleukin 2 receptor antagonist (IL2RA) induction in kidney transplants-the devil is in the detail. Transpl Int. 2019;32:794-6.
- [CrossRef] [PubMed] [Google Scholar]
- KDIGO clinical practice guideline for the care of kidney transplant recipients: A summary. Kidney Int. 2010;77:299-311.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin 2 receptor antagonists for renal transplant recipients: A meta-analysis of randomized trials. Transplantation. 2004;77:166-76.
- [CrossRef] [PubMed] [Google Scholar]
- Tailored rabbit antithymocyte globulin induction dosing for kidney transplantation. Transplant Direct. 2018;4:e343.
- [CrossRef] [PubMed] [Google Scholar]
- Rabbit anti-thymocyte globulin (rATG) versus IL-2 receptor antagonist induction therapies in tacrolimus-based immunosuppression era: A meta-analysis. Int Urol Nephrol. 2020;52:791-802.
- [CrossRef] [PubMed] [Google Scholar]
- Increased risk of rejection after basiliximab induction in sensitized kidney transplant recipients without pre-existing donor-specific antibodies-A retrospective study. Transpl Int. 2019;32:820-30.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing outcomes between antibody induction therapies in kidney transplantation. J Am Soc Nephrol. 2017;28:2188-200.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of IL2ra induction therapy in kidney transplantation using tacrolimus-and mycophenolate-based immunosuppression. Transplantation. 2010;90:639-44.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized double-blind study of immunoprophylaxis with basiliximab, a chimeric anti-interleukin-2 receptor monoclonal antibody, in combination with mycophenolate mofetil-containing triple therapy in renal transplantation. Transplantation. 2003;75:37-43.
- [CrossRef] [PubMed] [Google Scholar]
- Standard induction with basiliximab versus no induction in low immunological risk kidney transplant recipients: Study protocol for a randomized controlled trial. Trials. 2021;22:414.
- [CrossRef] [PubMed] [Google Scholar]
- Rabbit anti-thymocyte globulin for the prevention of acute rejection in kidney transplantation. Am J Transplant. 2019;19:2252-61.
- [CrossRef] [PubMed] [Google Scholar]
- Thymoglobulin versus basiliximab induction therapy in low-risk kidney transplant recipients: A single-center experience. Transplant Proc. 2018;50:1285-8.
- [CrossRef] [PubMed] [Google Scholar]
- Basiliximab does not reduce the early rejection incidence in high-risk kidney recipients under tacrolimus-based immunosuppression. Transplant Proc. 2008;40:2234-6.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of basiliximab induction in poorly matched living donor renal transplantation. Indian J Nephrol. 2013;23:409-12.
- [CrossRef] [PubMed] [Google Scholar]
- Induction therapies in live donor kidney transplantation on tacrolimus and mycophenolate with or without steroid maintenance. Clin J Am Soc Nephrol. 2015;10:1041-9.
- [CrossRef] [PubMed] [Google Scholar]
- Use and outcomes of induction therapy in well-matched kidney transplant recipients. Clin J Am Soc Nephrol. 2022;17:271-9.
- [CrossRef] [PubMed] [Google Scholar]
- [Effects of donor age and gender on early acute rejection episode in living related donor kidney transplantation] Zhonghua Yi Xue Za Zhi. 2008;88:3407-10.
- [PubMed] [Google Scholar]
- Living donor kidney transplantation: the effects of donor age and gender on short-and long-term outcomes. Transplantation. 2007;83:600-6.
- [CrossRef] [PubMed] [Google Scholar]
- Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev. 2017;1:CD004759. doi: 10.1002/14651858.CD004759.pub2
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of thymoglobulin and basiliximab in kidney transplant patients at high risk for acute rejection and delayed graft function. Exp Clin Transplant. 2013;11:310-4.
- [CrossRef] [PubMed] [Google Scholar]







